Abstract
Phytochemicals (PHT) are a large group of biologically active plant chemicals that may have positive effects on human health such as immune system stimulation, down regulation of inflammatory responses, radical scavenging activities, cell repair function, and antibacterial and antiviral activity. In this proof of principle 6 months study, the effects of supplementing a PHT mix, Phyto V7, to HIV-1 seropositive individuals and AIDS patients were examined. Individuals with CD4+ T-cells below 350 counts/mm3were assigned to one of the following treatments: CG1 - no treatment, CG2 - only highly active antiretroviral treatment (HAART), TG1 - only Phyto V7, and TG2- both Phyto V7 and HAART. After 3 months of treatment there were approximately (-)1%, 1%, 2% and 4% increase in the mean weight of the CG1, CG2, TG1 and TG2 groups, respectively. The tendency for the body mass index (BMI) was similar. The CD4+ counts increased by 13%, 39%, 53% and 35%, respectively. Similar trends were noted after 6 months with 2%, 79%, 53% and 69% increases in the CD4+ counts, respectively. There was a significant reduction in viremia only in groups receiving HAART. Overall better results were obtained in the group of patients receiving both HAART and Phyto V7, in which the mean weight increased by 5.7% and the CD4+ T-cell counts increased by 69% after 6 months. This study indicates that providing Phyto V7 to HIV-1 seropositive individuals and AIDS patients, receiving or not receiving HAART, improves their physical wellbeing and CD4+ counts, enabling them to cope better with the viral infection.
Author Contributions
Academic Editor: luca gallelli, University of Catanzaro
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2015 Wernik JR., et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
HIV infection and AIDS are endemic in many malnourished populations. A balanced nourishing diet is fundamental for healthiness and survival for all individuals, including for HIV-infected individuals. Tuberculosis and diarrhea, which occur in many HIV infected individuals, cause by themselves appetite loss, atrophy and weight loss. In HIV-infected individuals, the body energy requirements are higher than in non-HIV infected individuals1, 2, 3, 4, 5. Following HIV infection, before and after the onset of AIDS, including in AIDS patients receiving highly active antiretroviral treatment (HAART), the energy requirements needed to preserve the body weight increase by 20% to 30%6, 7, 8. In view of the above, the World Health Organization has recommended the inclusion of micronutrients (MMN) administration in any treatment protocol for HIV-infected individuals at any stage of their disease, including during pregnancy and lactation and for children6.
Almost in all randomized controlled trials that studied the effects of MMN supplementation found increased CD4+ T-cell counts or reduced mortality in the group of HIV-infected persons receiving MMN as compared to the HIV-infected persons receiving placebo9, 10, 11, 12, 13, 14, 15. Different MMN interventions have been evaluated in the various trials conducted and the conclusion from all these studies is that MMN supplementation confers clear clinical benefits to HIV-infected individuals, including to pregnant women and their offspring, regardless of their clinical stage and use of Antiretroviral Therapy (ART)15.
Phytochemicals, chemical compounds that occur naturally in plants, serve as micronutrients. Importantly, some phytochemicals also have additional important beneficial properties, as demonstrated in several clinical studies. For example, some phytochemicals possess radical scavenging activities16 some stimulate nonspecific immunity17 some down regulate inflammatory diseases18 and some have anti-hepatotoxic, anti-lithic, anti-hypertensive, and anti-hepatitis properties19. Interestingly, some phytochemicals demonstrate potent anti-HIV in vitro activity, especially against the HIV-1 protease and integrase, and against gp41 acting as entry inhibitors16, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28.
Phyto V7 is a specific mix of phytochemicals that has been found to have immune-stimulating properties. This was demonstrated in two separate studies. In the first one, administration of Phyto V7 to chicks vaccinated against Newcastle Disease Virus resulted in enhanced humoral immune responses against the virus 29. In the second study, administration of Phyto V7 to women, infected with Human Papilloma Virus (HPV) and with preneoplastic cervical lesions, resulted in enhanced cervical in situ cellular immune responses and increased clearance of HPV30.
In the current study we studied the clinical and immunological effects of the administration of Phyto V7 on HIV-1 seropositive individuals and AIDS patients, in order to determine if this phytochemical complex may be an important nutritional component to be given to these populations.
Experimental Procedure
This was a prospective, controlled clinical trial designed to determine the effect of Phyto V7 supplementation on HIV-1 disease progression in HIV-infected individuals receiving or not receiving HAART. It consisted of an Intervention Test Group and a Non Intervention Control Group (Figure 1). The Test Group received Phyto V7 and was divided into 2 subgroups: with and without HAART. The Control Group did not receive Phyto V7 and was also divided into 2 subgroups: with and without HAART.
Material and Methods
Enrollment took place between December 2009 and February 2010 in Hospital de Clínicas, Universidad Nacional de Córdoba, city of Cordoba, Argentina. Clinical interview, anthropometric and hematological analysis were performed prior to enrollment. Only diagnosed HIV-1 seropositive patients with less than 350 CD4+ T-cells per mm³, who had never received ART, were enrolled. Individuals with co-current infections or positive to hepatitis antibodies were excluded. Written informed consent was obtained from all study participants after explaining the trial aims and specifics in detail. Randomization to each of the detailed groups above was guided by the expressed will of the patient. A total of 28 patients were enrolled and divided into 4 groups as detailed in Figure 1: Control Group 1 (CG1; no Phyto V7, no ART); Control Group 2 (CG2; no Phyto V7, HAART); Test Group 1 (TG1; Phyto V7, no ART); and Test Group 2 (TG2; Phyto V7, HAART). The mean and age range, sex, weight and other characteristics of the enrolled patients per group are detailed in Table 1. The concomitant infections and HAART regimens of each of the patients are detailed in Table 2. In the Test Groups 1 and 2, the patients were requested to consume 2 tablets of Phyto V7 every 8 hours daily for the duration of the trial. Each Phyto V7 tablet contained 760 mg of the following phytochemicals: flavonols (Kaempferol, Quercetin), flavones (Apigenin, Luteolin), hydroxy-cinnamic acids (ferrulic acid), carotenoids (Lutein, Lycopene, Beta carotenne) andorganosulfur compounds, all from vegetal origin31. Cross-sectional clinical and laboratory studies were conducted every 3 months. The CD4 lymphocyte count was measured by conventional flow cytometry. Plasma HIV-1 RNA was measured using an ultraquantitative polymerase chain reaction assay with a lower limit of quantification of 50 copies/mL.
Table 1. Characteristics of Patients at the Beginning of the Trial| CG1 | CG2 | TG1 | TG2. | |
| Number | 7 | 8 | 7 | 6 |
| Sex (Male/Female) | 6/1 | 5/3 | 5/2 | 5/1 |
| Age (mean±SD) male | 33±8 | 37±8 | 31±7 | 43±10 |
| Age (mean±SD) female | 54 | 42±9 | 28±3 | 46 |
| Age (range) male | 26-44 | 29-49 | 22-39 | 33-57 |
| Age (range) female | 54 | 33-51 | 25-30 | 46 |
| BMI male | 22±1 | 22±1 | 24±2 | 21±3 |
| BMI female | 21 | 22±2 | 22±0.2 | 23 |
| CDC Classification | A2 all | A2 all | A2-6, A3-1 | A2-5, C2-1 |
| CD4 cells/mm3(mean±SD) | 295±32 | 271±42 | 276±38 | 233±74 |
| CD4 cells/mm3 (range) | 237-336 | 203-350 | 175-329 | 93-298 |
| Ln Viral load (mean±SD) | 10.11±1.22 | 10.59±0.7 | 10.78±0.96 | 10.35±1.99 |
| Patient # | Group | Phyto V7 | HAART Regimen | Concomitant infections |
|---|---|---|---|---|
| 1 | CG1 | None | None | Asymptomatic |
| 2 | CG1 | None | None | Asymptomatic |
| 3 | CG1 | None | None | Candidiasis |
| 4 | CG1 | None | None | Asymptomatic |
| 5 | CG1 | None | None | Microsporidium |
| 6 | CG1 | None | None | Asymptomatic |
| 7 | CG1 | None | None | Asymptomatic |
| 8 | CG2 | None | Lamivudine/Zidovudine/Efavirenz | Asymptomatic |
| 9 | CG2 | None | Lamivudine/Abacabir/Efavirenz | Tuberculosis |
| 10 | CG2 | None | Lamivudine/Zidovudine/Nevirapine | Asymptomatic |
| 11 | CG2 | None | Lamivudine/Zidovudine/Nevirapine | Asymptomatic |
| 12 | CG2 | None | Abacabir/Tenofovir/Nevirapine | Asymptomatic |
| 13 | CG2 | None | Lamivudine/Zidovudine/Nevirapine | Asymptomatic |
| 14 | CG2 | None | Lamivudine/Zidovudine/Nevirapine | Pneumocystis Jiroveci |
| 15 | CG2 | None | Abacabir/Tenofovir/Nevirapine | Asymptomatic |
| 16 | TG1 | Yes | None | Asymptomatic |
| 17 | TG1 | Yes | None | Asymptomatic |
| 18 | TG1 | Yes | None | Asymptomatic |
| 19 | TG1 | Yes | None | Asymptomatic |
| 20 | TG1 | Yes | None | Tuberculosis |
| 21 | TG1 | Yes | None | Asymptomatic |
| 22 | TG1 | Yes | None | Asymptomatic |
| 23 | TG2 | Yes | Lamivudine/Zidovudine/Nevirapine | Asymptomatic |
| 24 | TG2 | Yes | Lamivudine/Didanosine/Nevirapine | Asymptomatic |
| 25 | TG2 | Yes | Lamivudine/Zidovudine/Efavirenz | Epigastric distress |
| 26 | TG2 | Yes | Abacabir/Tenofovir/Nevirapine | Asymptomatic |
| 27 | TG2 | Yes | Abacabir/Tenofovir/Nevirapine | Candidiasis |
| 28 | TG2 | Yes | Lamivudine/Zidovudine/Nevirapine | Asymptomatic |
Statistical Analysis
The Statistical Analysis Plan included the group mean, median and range analysis. The standard deviation (SD <10 and> 90) was performed by comparing the mobility of indicators in each case with longitudinal monitoring, average and increased by cuttings. For n = 28, validation sample was subjected to Yates Chi Square, giving a confidence interval of 95% (CI = 95) with a value α = 0.05, P = 0.2432. The delta % change in the weight or BMI of the patients after 3 and 6 months was calculated using the following equation (Weight or BMI at 3 or 6 months/Weight or BMI at baseline) x100-100. A Paired T-test was used to compare the means before and after treatment within groups. A Student T-test and/or a Mann-Whitney Rank Sum Test was used to compare between the changes in the weight, BMI and CD4+ T-cell counts between the groups. ANOVA of Kruskal & Wallis analyses and Conover post-test were used to compare the patient’s characteristics at the onset of the trial. SigmaPlot 12.0 software was used to conduct the above statistical tests.
Results
Characteristics of Trial Participants at Recruitment
Twenty eight patients were recruited to the study and assigned into 4 groups as detailed in Figure 1, according to the patients expressed will. According to the CDC Classification System for HIV infection, 26 patients were classified A2 patients, one A3 and one with more severe symptoms as C2 (Table 1). The concomitant infections and HAART regimens for each group are also detailed in Table 2. The overall patient’s characteristics were similar between all 4 groups at enrollment. These included physical characteristics such as the age, weight, Body Mass Index (BMI) and height of the patients, and similar CDC classification (most patients were A2), viremia and CD4+ T-cell counts. Data points were collected from all patients for baseline, 3 and 6 months.
Patient’s Physical Status
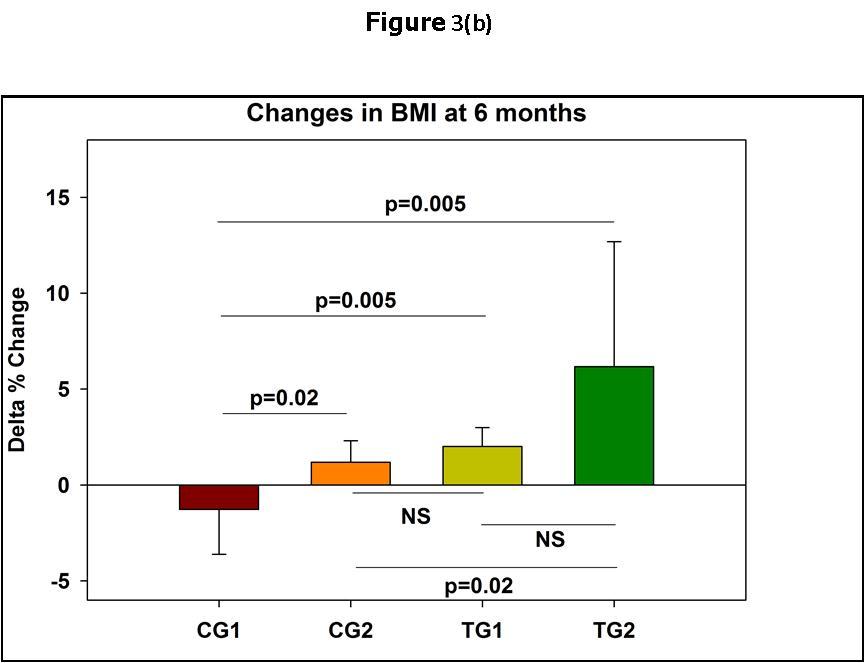
After 3 months from the commencement of the Trial it became clear that there were obvious differences between the physical wellbeing of the patients in the different groups. This was especially noticeable in the increase in the weight of the patients (Table 3). While in the CG1 there was a mean decrease in the weight of the patients of 1%, and in the CG2 patients there was a slight 1% non-significant increase in the weight of the patients as compared to the CG1, in the TG1and TG2 groups, there was a 2% and 4% statistically significant increases in the mean weights of the patients as compared to the reference CG1 (Figure 2a). The differences in the weight changes were even more noticeable at 6 months, especially in the TG2 group (Figure 2b and Figure 2c). Very similar results were obtained with the Body Mass Indexes (Figure 3). In Control Group 1, that did not receive any treatment, there were no improvement in the CDC classification of the Patients at 3 and 6 months after the commencement of the Trial. In Control Group 2 that received ART only, in 0/8 and 5/8 of the patients improvements in their CDC classifications occurred at 3 and 6 months, respectively. In Test Group 1 that received PHT only, in 2/7 and 3/7 of the patients improvements in their CDC classification occurred at 3 and 6 months, respectively. In Test Group 2 that received ART and PHT, in 2/6 and 4/6 of the patients, improvements in their CDC classification occurred at 3 and 6 months, respectively.
Table 3. Mean, median and SDs of weights and BMI at 3 and 6 months of treatment| Months | CG1 | CG2 | TG1 | TG2 | ||
| Weight | Mean | 0 | 65 | 70.37 | 69.43 | 67.67 |
| 3 | 64.27 | 70.76 | 70.54 | 70 | ||
| 6 | 64.24 | 71.27 | 70.8 | 71.55 | ||
| Median | 0 | 65 | 68.5 | 67 | 64 | |
| 3 | 65.4 | 69 | 67.3 | 67.5 | ||
| 6 | 65 | 69.3 | 67.7 | 71.5 | ||
| SDs | 0 | 5.38 | 9.53 | 10.2 | 12.1 | |
| 3 | 5.86 | 9.76 | 10.39 | 11.49 | ||
| 6 | 6.37 | 10.2 | 10.39 | 11.14 | ||
| BMI | Mean | 0 | 21.69 | 22.3 | 22.86 | 21.44 |
| 3 | 21.45 | 22.42 | 23.23 | 22.18 | ||
| 6 | 21.43 | 22.57 | 23.32 | 22.68 | ||
| Median | 0 | 21.72 | 22.56 | 21.97 | 21.39 | |
| 3 | 22.04 | 22.74 | 22.4 | 21.9 | ||
| 6 | 22.1 | 22.82 | 22.51 | 22.87 | ||
| SDs | 0 | 1.15 | 1.43 | 1.73 | 2.43 | |
| 3 | 1.41 | 1.51 | 1.82 | 2.11 | ||
| 6 | 1.52 | 1.63 | 1.81 | 2 |
Figure 2.Changes in the patients weight from baseline at 3 and 6 months of the zommencement of the Trial. In (a) and (b) the means and standard deviations are shown. In (c) the median and standard errors are shown. * p<0.05; ** p<0.01 per group as compared to time 0.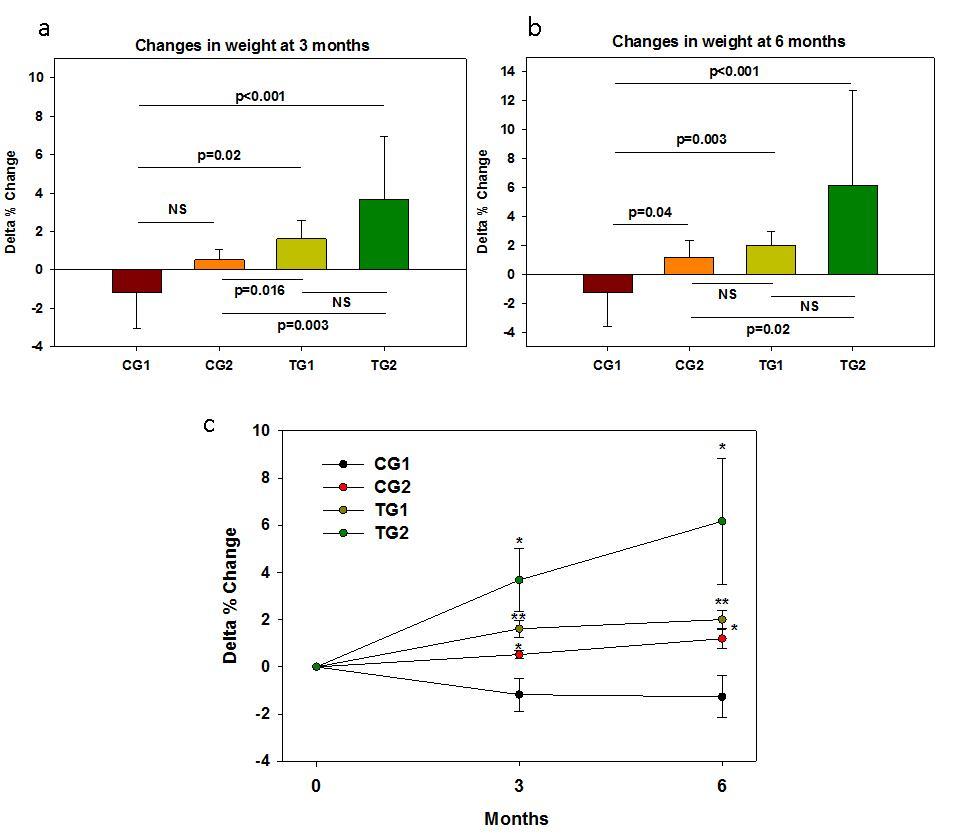
Figure 3.Changes in the patients BMI from baseline at 3 and 6 months of the commencement of the Trial.In (a) and (b) the means and standard deviations are shown. In (c) the median and standard errors are shown. * p<0.05; ** p<0.01 per group as compared to time 0.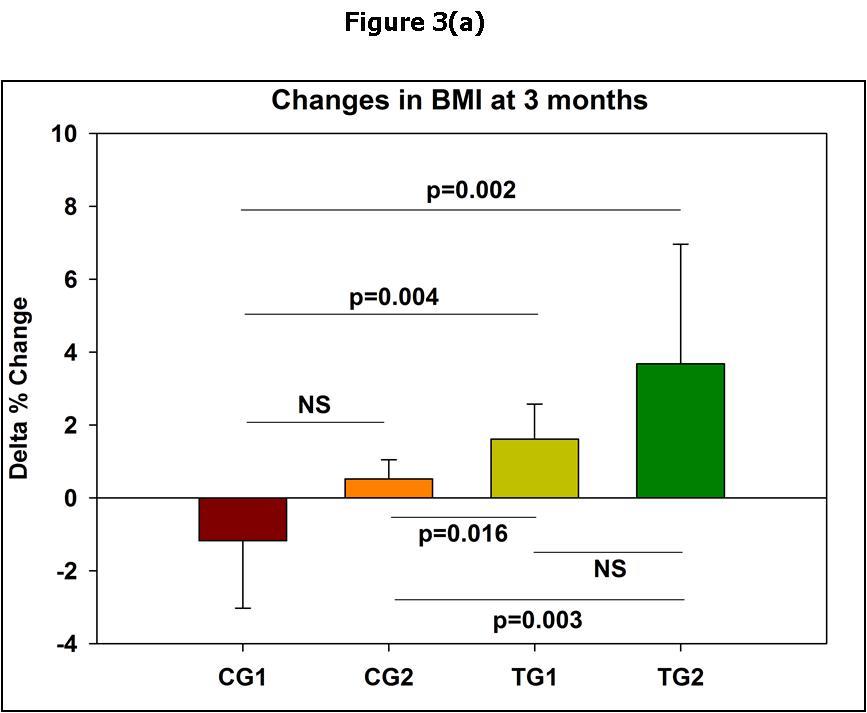
Viremia
The patients in all 4 groups had similar significant high viral loads with no statistically significant differences in the group mean viremia at the beginning of the Trial (Table 1). As depicted in Figure 4 and detailed in Table 4, the mean viral load in the Control Group 1 that did not receive ART or PHT did not change significantly over the 6 months Trial. Similarly, there were no changes in the Test Group 1 that received PHT but did not receive ART. In contrast, the mean viral load in the patients of the Control Group 2, who received ART, was below detectable levels already after 3 months of treatment and remained below detectable levels also at 6 months of treatment. In the test Group 2, which received both ART and PHT, there was a reduction in the viral load below detectable levels at 3 months in all patients but one. After changing the antiretroviral medication to this patient, from the 3rd month of the commencement of the Trial and onward, his viral load was reduced to undetectable levels. Also in this group of patients no viral load was detected at 6 months.
Figure 4.Viral loads at baseline and after 3 and 6 months after the commencement of the Trial.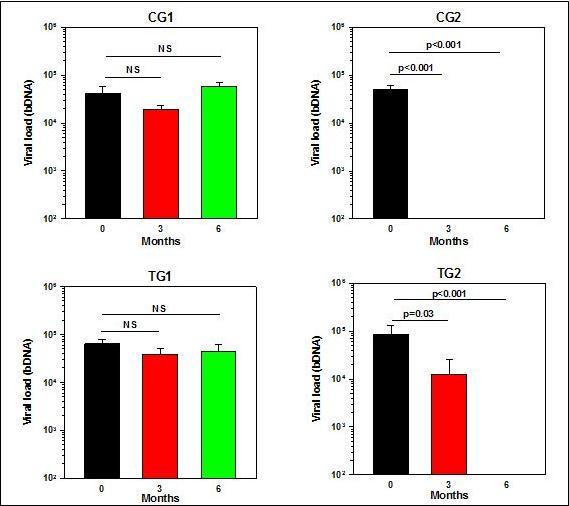
| Months | CG1 | CG2 | TG1 | TG2 | ||
| Viremia (ln) | Mean | 0 | 10.11 | 10.59 | 10.78 | 10.35 |
| 3 | 9.79 | 3.91* | 9.58 | 5.34 | ||
| 6 | 10.77 | 3.91 | 9.47 | 3.91 | ||
| Median | 0 | 10.16 | 10.42 | 11.16 | 10.84 | |
| 3 | 9.78 | 3.91 | 10.52 | 3.91 | ||
| 6 | 11.04 | 3.91 | 10.04 | 3.91 | ||
| SDs | 0 | 1.22 | 0.7 | 0.96 | 1.99 | |
| 3 | 0.44 | 0.0 | 2.58 | 3.19 | ||
| 6 | 0.81 | 0.0 | 2.64 | 0.0 | ||
| CD4+ T-cells | Mean | 0 | 295 | 271 | 276 | 233 |
| 3 | 335 | 378 | 424 | 315 | ||
| 6 | 300 | 485 | 428 | 394 | ||
| Median | 0 | 297 | 266 | 274 | 255 | |
| 3 | 351 | 377 | 434 | 330 | ||
| 6 | 328 | 495 | 401 | 402 | ||
| SDs | 0 | 32.2 | 42.2 | 38.5 | 73.7 | |
| 3 | 68 | 107.6 | 172.5 | 173.6 | ||
| 6 | 75.5 | 141.1 | 119.1 | 170.8 | ||
CD4+ T-cell Counts
As depicted in Figure 5 and detailed in Table 4, no significant changes in CD4+ T-cell counts occurred in all patients that belonged to the Control Group 1 during the 6 months trial. In the Control Group 2 that received ART there was a statistically significant increase from baseline in the CD4+ T-cell counts at 3 months (39%; p=0.02), which further increased at 6 months (79%; p<0.001). Impressively, also in the Test Group 1, who did not take any ART but only PHT, there was a significant increase of ~53% in the CD4+ T-cell counts at 3 and 6 months of treatment (p<0.05). Similar trend was noted in Test Group 2, who received both ART and PTH with 35% and 69% of increase in the mean CD4+ T-cell counts at 3 and 6 months, although in this group no statistical significance was reached (p=0.06).
Figure 5.CD4 counts at baseline and after 3 and 6 monthsafter the commencement of the Trial.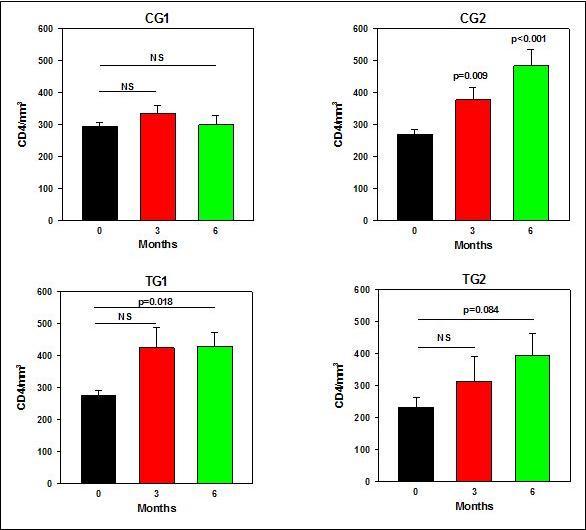
Discussion
This study demonstrates that the oral administration of a phytochemical complex (Phyto V7) is beneficial to HIV-1 seropositive individuals and AIDS patients, whether receiving antiretroviral therapy or not. There were quantitative improvements in the patients receiving Phyto V7 in their weight, BMI and CD4+ T-cell counts. While there was a mean decrease in the weight and BMI of the patients that did not receive any treatment during the trial, in the patients that received only Phyto V7 there was a significant increase in the weight and BMI both after 3 and 6 months of the study. Interestingly, in the patients that received only ARV treatment, there was a statistical significant increase in weight and BMI only after 6 months of treatment, as compared to the patients that did not receive any treatment. The most notorious increases in weight and BMI were noted in the patients that received both ARV treatment and Phyto V7. Meaningfully, there was a clear statistical difference between the increase in weight and BMI in those patients that received ARV treatment only and those that received the ARV and also the phytochemicals both at 3 and 6 months (Figure 2 and Figure 3), indicating the significant contribution of the phytochemicals to the well-being of the patients. Outstandingly, there was also clear improvement in the CD4+ T-cell counts of the group of patients that received Phyto V7 only as compared to those that did not receive any treatment (Figure 5), which was similar to the increase in CD4+ T-cell counts that occurred in the group of patients that received antiretroviral treatment only. The medical improvement in the patients receiving Phyto V7 was very noticeable according to the treating doctor’s impression and the patient’s feedback (appetite, reduction of diarrhea). While it has been reported that some phytochemicals possess potent anti-HIV in vitro activity16, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, apparently their beneficial effects cannot be explained by their direct activity on the virus, as there were no decreases in viremia in the patients that received the phytochemicals only. The results of this study are in accordance with the dramatic improvement in the physical status of a small cohort of 9 terminal AIDS patients following 45 days of administration of Phyto V732 and with the significant improvement in the well-being of 199 HIV-1 infected individuals, who were not undergoing antiretroviral treatment and were recruited as part of the Uruguay National Program of AIDS, which received only a daily administration of Phyto V7 for a period of 90 consecutive days31.
The HAART regimens used in this trial were based on the recommended Argentinian Ministry of Health guidelines at the time the trial was conducted. Those regimens as well as other HAART regimens, when taken with good compliance, can effectively reduce the viral loads to undetectable levels and significantly improve prognosis and well-being of the patients, as indeed was the case in the group of patients in this study that only received the antiretroviral therapy. However, HAART may cause also significant adverse effects, such as lipodystrophy, dyslipidaemia, cardiovascular complications, central and peripheral nervous system disturbances and insulin resistance33, 34, 35, 36. HAART may be very problematic to certain populations, such as pregnant women and children37, 38. The large number of pills needed for HAART also leads to significant problems of compliance and development of resistant virus39. HAART is not implemented in many resource-limited regions where the AIDS epidemic is rampant due to its high cost. Furthermore, the spectrum of adverse effects related to HAART in developing countries may be even more deleterious and hard to treat because of the high prevalence of conditions such as anemia, malnutrition, and tuberculosis and frequent initial presentation with advanced HIV disease40. Taken together, there is a rational for postponing the administration of HAART to HIV-1 infected patients as much as possible, but not too late before the CD4+ T-cell counts are too low 41.
As indicated in this study, in patients that did not receive antiretroviral treatment, the administration of Phyto V7 resulted in an increase in their CD4+ T-cell counts, weight and BMI, indicating that supplementation of this phytochemical mix may improve the capacity of HIV-1 infected individuals to cope with the viral infection and potentially delaying the need to treat them with HAART, postponing the potential complications associated with HAART treatment.
HAART treatment taken with good compliance in most cases results in viremia suppression, immune reconstitution, and reduction in incidence and severity of opportunistic diseases and death42. Similar results were obtained in this study. However, in many HIV-1 infected individuals and AIDS patients undergoing HAART treatment, there is no reconstitution of their immune system, and the CD4+ T-cell counts do not increase even if full plasma viral load suppression is achieved43. This may be further exasperated in AIDS/tuberculosis patients, AIDS patients that already suffer from opportunistic infections, or are co-infected with other parasites, such as helminthes or other viral infections, as is the case in many African countries. It would be very significant if in these individuals the supplementation with phytochemicals would increase their CD4+ T-cell counts. This would be a much simpler associated safe treatment than immune therapies being explored today like cytokine therapies, therapeutic immunization, monoclonal antibodies, immune-modulating drugs, nanotechnology-based approaches and radio immune therapy 44, 45.
What are the mechanisms by which the phytochemicals benefit HIV-1 seropositive and AIDS patients is not yet clear and should be elucidated. While it has been reported that some phytochemicals possess potent anti-HIV in vitro activity16, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, apparently their beneficial effects cannot be explained by their direct activity on the virus, as there were no decreases in viremia in the patients that received the phytochemicals only (Figure 4). In the HAART and PHT co-treatment group (TG2), when looking at the mean viremia of the group (Figure 4), the impression that PHT counteracts the effect of HAART could be reached. However, as explained in the Results section, in the TG2 group there were 6 individuals, all of which had high viral loads at the onset of the trial. Following the HAART treatment and PHT co-treatment in 5 out of the 6 patients, no viral load was detected at the 3 months period (less than ln 3.9, i.e. less than 50 viral RNA copies/ml). Unfortunately, in one individual the viremia remained very high (62685 viral RNA copies/ml) at 3 months examination. It turned out that this particular patient had a resistant virus and only after changing the antiretroviral medication, from the 3rd month of the commencement of the Trial and onward, the HAART treatment was efficacious. This indicates that the PHT co-treatment does not interfere with antiviral activity of HARRT therapy.
Part of their positive effect can be explained as serving as micronutrients, having radical scavenging activities16, stimulating nonspecific immunity17, and by down regulating inflammatory responses18. Importantly, administration of Phyto V7 to 33 women infected with Human Papilloma Virus (HPV) and with preneoplastic cervical lesions, resulted in enhanced cervical in situ cellular immune responses and increased clearance of HPV30. Additionally, enhancement of antibody titers against Newcastle Disease Virus occurred in vaccinated chicks following administration of Phyto V729, further supporting the notion that Phyto V7 has immune-stimulatory properties.
Additional studies should be performed to support the notion that supplementation of phytochemicals to HIV-1 infected patients is beneficial, and to elucidate their mechanism of action. This should be done with larger cohorts of HIV-1 infected individuals and AIDS patients. In the current study only patients with less than 350 CD4+ T-cells per mm3 were recruited. Future studies should examine individuals with significantly higher CD4+ T-cell counts not receiving HAART as well as individuals with very low CD4 T-cell counts receiving or not receiving HAART. Future studies should also include patients receiving just multiple micronutrients supplementation (MMN) and compare them with patients receiving just phytochemicals. If proven the significant added value of phytochemicals over MMN, current recommendations, like the UN requested of inclusion of MMN for treatment of HIV carriers and AIDS patients at any stage of their disease, should be revised to include phytochemicals.
References
- 1.Hommes M J, Romijn J A, Godfried M H, Schattenkerk J K, Buurman W A. (1990) Increased resting energy expenditure in human immunodeficiency virus-infected men Metabolism. 39, 1186-1190.
- 2.Melchior J C, Raguin G, Boulier A, Bouvet E, Rigaud D. (1993) Resting energy expenditure in human immunodeficiency virus-infected patients: comparison between patients with and without secondary infections. , Am J Clin Nutr 57, 614-619.
- 3.Melchior J C, Salmon D, Rigaud D, Leport C, Bouvet E. (1991) Resting energy expenditure is increased in stable, malnourished HIV-infected patients. , Am J Clin Nutr 53, 437-441.
- 4.Batterham M J. (2005) Investigating heterogeneity in studies of resting energy expenditure in persons with HIV/AIDS: a meta-analysis. , Am J Clin Nutr 81, 702-713.
- 5.Barron M A, Makhija M, Hagen L E, Pencharz P, Grunebaum E. (2011) Increased resting energy expenditure is associated with failure to thrive in infants with severe combined immunodeficiency. , J Pediatr 159, 628-632.
- 6. (2003) World Health Organization.Nutrient requirements for people living with HIV/AIDS: report of a technical consultation.
- 7.Sutinen J, Yki-Jarvinen H. (2007) Increased resting energy expenditure, fat oxidation, and food intake in patients with highly active antiretroviral therapy-associated lipodystrophy. , Am J Physiol Endocrinol Metab 292, 687-692.
- 8.Shevitz A H, Knox T A, Spiegelman D, Roubenoff R, Gorbach S L. (1999) Elevated resting energy expenditure among HIV-seropositive persons receiving highly active antiretroviral therapy. , AIDS 13, 1351-1357.
- 9.Fawzi W W, Msamanga G I, Spiegelman D, Wei R, Kapiga S. (2004) A randomized trial of multivitamin supplements and HIV disease progression and mortality. , N Engl J Med 351, 23-32.
- 10.Jiamton S, Pepin J, Suttent R, Filteau S, Mahakkanukrauh B. (2003) A randomized trial of the impact of multiple micronutrient supplementation on mortality among HIV-infected individuals living in Bangkok. , AIDS 17, 2461-2469.
- 11.Kaiser J D, Campa A M, Ondercin J P, Leoung G S, Pless R F. (2006) Micronutrient supplementation increases CD4 count in HIV-infected individuals on highly active antiretroviral therapy: a prospective, double-blinded, placebo-controlled trial. , J Acquir Immune Defic Syndr 42, 523-528.
- 12.Kawai K, Kupka R, Mugusi F, Aboud S, Okuma J. (2010) A randomized trial to determine the optimal dosage of multivitamin supplements to reduce adverse pregnancy outcomes among HIV-infected women in Tanzania Am. , J Clin Nutr 91, 391-397.
- 13.Semba R D, Kumwenda J, Zijlstra E, Ricks M O, van L M. (2007) Micronutrient supplements and mortality of HIV-infected adults with pulmonary TB: a controlled clinical trial. , Int J Tuberc Lung Dis 11, 854-859.
- 14.Forrester J E, Sztam K A. (2011) Micronutrients in HIV/AIDS: is there evidence to change the WHO2003recommendations?Am. , J Clin Nutr 94, 1683-1689.
- 15.Siegfried N, Irlam J H, Visser M E, Rollins N N. (2012) Micronutrient supplementation in pregnant women with HIV infection Cochrane Database Syst Rev.3,CD009755.
- 16.Wang X, Liu Z, Qiao W, Cheng R, Liu B. (2012) Phytochemicals and biological studies of plants from the genus Balanophora Chem Cent. , J 6, 79.
- 17.Sun L Z, Currier N L, Miller S C. (1999) The American coneflower: a prophylactic role involving nonspecific immunity. , J Altern Complement Med 5, 437-446.
- 18.Aggarwal B B, Shishodia S. (2004) Suppression of the nuclear factor-kappaB activation pathway by spice-derived phytochemicals: reasoning for seasoning. , Ann N Y Acad Sci 1030, 434-441.
- 19.Bagalkotkar G, Sagineedu S R, Saad M S, Stanslas J. (2006) Phytochemicals from Phyllanthus niruri Linn and their pharmacological properties: a review. , J Pharm Pharmacol 58, 1559-1570.
- 20.Berginc K, Trdan T, Trontelj J, Kristl A. (2010) HIV protease inhibitors: garlic supplements and first-pass intestinal metabolism impact on the therapeutic efficacy Biopharm Drug Dispos. 31, 495-505.
- 21.Mushi N F, Mbwambo Z H, Innocent E, Tewtrakul S. (2012) Antibacterial, anti-HIV-1 protease and cytotoxic activities of aqueous ethanolic extracts from Combretum adenogonium Steud Ex A Rich (Combretaceae). , BMC. Complement Altern Med 12, 163.
- 22.Xia C L, Mao Q C, Li R M, Chen Z P, Jiang S B. (2010) [Study of the mechanism of caffeoyl glucopyranoses in inhibiting HIV-1 entry using pseudotyped virus system] Nan Fang Yi Ke Da Xue Xue Bao. 30, 720-723.
- 23.Bunluepuech K, Sudsai T, Wattanapiromsakul C, Tewtrakul S. (2011) Inhibition on HIV-1 integrase activity and nitric oxide production of compounds from Ficus glomerata Nat Prod Commun. 6, 1095-1098.
- 24.Tewtrakul S, Subhadhirasakul S, Cheenpracha S, Karalai C. (2007) . HIV-1 protease and HIV-1 integrase inhibitory substances from Eclipta prostrata Phytother Res 21, 1092-1095.
- 25.Tewtrakul S, Itharat A, Rattanasuwan P. (2006) Anti-HIV-1 protease- and HIV-1 integrase activities of Thai medicinal plants known as Hua-Khao-Yen. , J Ethnopharmacol 105, 312-315.
- 26.Cheenpracha S, Karalai C, Ponglimanont C, Subhadhirasakul S, Tewtrakul S. (2006) Anti-HIV-1 protease activity of compounds from Boesenbergia pandurata Bioorg Med Chem. 14, 1710-1714.
- 27.Tewtrakul S, Miyashiro H, Nakamura N, Hattori M, Kawahata T. (2003) . HIV-1 integrase inhibitory substances from Coleus parvifolius Phytother Res 17, 232-239.
- 28.Zhang C F, Nakamura N, Tewtrakul S, Hattori M, Sun Q S. (2002) Sesquiterpenes and alkaloids from Lindera chunii and their inhibitory activities against HIV-1 integrase Chem Pharm Bull (Tokyo). 50, 1195-1200.
- 29.Perelman D, Goldman W F, Wernik Borkow, G. (2013) Enhancement of antibody titers against newcastle disease virus in vaccinated chicks by administration of Phyto V7. , Journal of Vaccines and Vaccination 4, 7-8.
- 30.Goldman W F, Wernik R, Carmen-Elias A, Borkow G. (2014) Immunomodulating effect of Phyto V7 in preneoplastic cervical lesions Med. , J Obstet Gynecol 2, 1038-1042.
- 31.Wernik J R, Priore J L, Goldman W F, Carmen-Elias A, Borkow G. (2015) . Improvement in Well-being of HIV-1 Infected Individuals and AIDS Patients Following Administration of the Phytochemical Complex "Phyto V7" WJCID 5, 44-50.
- 32.DMM Lavandera, Jiminian FAC, Wernik R, Goldman W F, Borkow G. (2013) Dramatic improvement in physical well-being of terminal AIDS patients following administration of phytochemicals World. , Journal of AIDS 3, 287-293.
- 33.Garg H, Joshi A, Mukherjee D. (2013) . , Cardiovascular Complications of HIV Infection and Treatment Cardiovasc Hematol Agents. Med Chem 11, 58-66.
- 34.Husstedt I W, Reichelt D, Neuen-Jakob E, Hahn K, Kastner F. (2009) [Highly active antiretroviral therapy of neuro-AIDS Side effects on the nervous system and interactions]Nervenarzt80,1133-8:. 1140.
- 35.Stankov M V, Behrens G M. (2007) HIV-therapy associated lipodystrophy: experimental and clinical evidence for the pathogenesis and treatment Endocr Metab Immune Disord Drug Targets. 7, 237-249.
- 36.Haugaard S B. (2006) Toxic metabolic syndrome associated with. , HAART Expert Opin Drug Metab Toxicol 2, 429-445.
- 37.Senise J F, Castelo A, Martinez M. (2011) Current treatment strategies, complications and considerations for the use of HIV antiretroviral therapy during pregnancy. , AIDS Rev 13, 198-213.
- 38.Shah C A. (2007) Adherence to high activity antiretrovial therapy (HAART) in pediatric patients infected with HIV: issues and interventions Indian. , J Pediatr 74, 55-60.
- 39.Zhan P, Liu X. (2009) Designed multiple ligands: an emerging anti-HIV drug discovery paradigm Curr Pharm Des. 15, 1893-1917.
- 40.Subbaraman R, Chaguturu S K, Mayer K H, Flanigan T P, Kumarasamy N. (2007) Adverse effects of highly active antiretroviral therapy in developing countries. , Clin Infect Dis 45, 1093-1101.
- 41.Perez-Molina J A, Diaz-Menendez M, Plana M N, Zamora J, Lopez-Velez R. (2012) Very late initiation of HAART impairs treatment response at 48 and 96 weeks: results from a meta-analysis of randomized clinical trials. , J Antimicrob Chemother 67, 312-321.
- 42.Autran B, Carcelain G, Li T S, Blanc C, Mathez D. (1997) Positive effects of combined antiretroviral therapy on. CD4+ T cell homeostasis and function in advanced HIV disease Science 277, 112-116.
- 43.Aiuti F, Mezzaroma I. (2006) Failure to reconstitute CD4+ T-cells despite suppression of HIV replication under. , HAART AIDS Rev 8, 88-97.