Evaluation Of Physicochemical Separation Characteristics of Pig Manure According to the Type of Solid-Liquid Separator Processes
Abstract
Objectives
The solid-liquid separation (SLS) process generally separates solid and liquid fractions in wastewater and livestock manure. The solid-liquid separation process is an essential pretreatment step for the recycling and purification of pig manure. This study has assessed the separation and/or reduction efficiency by various SLS processes used in pig farms.
Methods
Seven types of SLS processes (centrifuge, centrifuge (+coagulation agent), belt press (+ coagulation agent), drum screen, inclined screen, vibration screen, and screw press) were used on 11 pig farms and conducted a comparative analysis. As for the sample in this study, the pig raw manure before treatment, the separated liquid and solid manure after treatment of the SLS process collected, respectively. These samples were provided for pH, EC(electrical conductivity) moisture content, CODMn, BOD5, TN, TP, K, TS, SS, NaCl, and heavy metals analysis.
Results and Discussion
The belt press (+coagulation agent) process had the highest TS and SS reduction rate of 78.8% and 96.9%, respectively. The highest removal efficiency of TN and TP was41.0% and 94.2% by belt press (+coagulation agent) and centrifuge (+coagulation agent),respectively. The belt press (+coagulation agent) removed 59.4% and 66.0% of BOD5 and CODMn,respectively. The Zn and Cu were removed 100% and 98.6% by centrifuge (+coagulation agent).However, the drum screen, inclined screen, vibration screen, screw press, and centrifuge showed lower removal efficiency of nutrient contents, solids, Zn, and Cu than centrifugal and belt press processes with chemical coagulation.
Conclusions
The centrifugal and belt press separation processes that used chemical coagulation showed much more removal efficiency of nutrient contents, solids, and metals like Zn and Cu. Although SLS with chemical coagulants is an effective pre-treatment process for liquid manure treatment and helps removal effect for suspended solids, nutrients, and heavy metals, further studies are needed on how it affects biological or chemical processing processes that are linked.
Author Contributions
Copyright © 2023 Soo-Ryang KIM
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Citation:
Introduction
For a long time, livestock manure has been acknowledged as a valuable nutrient resource for plants and crops. However, livestock manure only contributes positively and replaces mineral and chemical fertilizers when it is used properly with a minimal loss of nutrients like nitrogen (N), phosphorus (P), and potassium (K)1,2). The P and K in livestock manure are similar to those in commercial fertilizers, but the N content is much lower than that in commercial fertilizers3). Excessive application of livestock manure as fertilizer and untreated and/or poorly treated manure causes nutrient leaching and runoff and ultimately pollutes surface and groundwater, salinizes semi-arid regions, results in toxic concentrations of heavy metals, and decreases soil aeration4,5,6,7). Before utilizing the manure as fertilizer, it should be treated properly to ensure its environmental safety with high fertilizer values. However, recovering the energy and nutrients from liquid manure is difficult because of its lower concentration of organic matter and nutrients8,9). Therefore, liquid manure separations become a key process in nutrient recovery strategies10).Pig manure is a combination of pig urine, feces, and water spillage, as well as remains of undigested food, antimicrobial drug residues, and pathogenic microorganisms11). It is commonly characterized by a high content of suspended solids, organic matter, phosphorus, and nitrogen16), which make it high in density and viscosity. Unlike cow and chicken manure, pig manure is more than 90% liquid, so it has limited options for treatment by composting. Solid–liquid separation (SLS) is part of organic and inorganic solids removal processes from manure slurry and most commonly separates manure into two streams known as the liquid and solid fractions. The SLS process accelerates the manure treatment practice and reduces the environmental impacts 12). According to Hjorth et al. (2010)3), the efficiency of an SLS system depends on (1) the chemical and physical properties of the slurry, (2) the desired end-products, and (3) the potential separation techniques, including pre-and post-treatments and combinations of different techniques. Typically, the separation efficiencies of mechanical SLS processes are between 34% and 68% 13,14). Because of the operational and environmental benefits, the SLS system is often accompanied by multi- step advanced manure treatment processes to improve subsequent treatment steps and achieve the environmental standards and nutrient recovery targets for livestock manure 11,15). For pig manure, SLS is a pre-treatment option that helps to separate N-rich liquid from P-rich solids and that allows the separated liquid to be used as a source of N to crops without over-supplying P17). Consequently, SLS in the manure pre-treatment process creates high processing efficiency by mobilizing biological processes due to the particulate solids. The high concentration of pig manure in the slurry state can reduce the processing load when solids are effectively removed (separated) by the SLS process. The SLS processes are mainly mechanical pre-treatment processes, but not all of them were designed to perform in the same fashion. The most common mechanical SLS systems are screen separators (inclined screen, vibrating screen, rotating screen), centrifuges (vertical and horizontal centrifuges), and presses (roller presses, belt presses, and screw presses). Landfilling and ocean dumping of livestock manure and food waste have been prohibited in South Korea since 2005 and 2012, respectively18). Therefore, several manure treatment technologies have been adopted in farm and public levels and they often consist of several treatment processes (SLS, composting, aeration, anaerobic digestion, liquid fertilization, and purification discharge) coupled with each other. This study evaluated the separation efficiency of BOD, COD, nutrients (N, P, and K), and heavy metals (Cu and Zn) of various solid-liquid separators used in pig farms.
Materials and Methods
Sampling Methods for Solid-Liquid Separation (SLS)
The sampling of the influent, effluent, and solid materials of the solid-liquid separation (SLS) was conducted after pre-operation of each device for 5 to 30 minutes, in accordance with the characteristics of the respective equipment. The sampling method adhered to specific regulations and guidelines regarding the turbidity sampling criteria and testing methods for the liquid fraction. For the sampling of the influent, effluent, and solid materials of the SLS, sampling was performed after a pre-operation period ranging from 5 to 30 minutes, depending on the characteristics of each device. In the case of liquid samples, such as the influent and effluent, a T-valve was installed at the inlet and outlet points to facilitate sample collection upon request (Please note that in pig farms where the installation of a T-valve was physically impractical, sampling was conducted directly from the inlet and outlet pipes). The liquid samples were collected by the operator after an arbitrary time determined based on on-site conditions. The influent and effluent samples were collected at the midpoint of the predetermined time interval. Regarding the collection of solid samples, the sampler designated an arbitrary time frame (ranging from 1 to 30 minutes) and employed an appropriate container to collect the entire discharge from the solid outlet of the SLS. The weight of the collected sample was measured on-site, followed by the extraction of a portion of the solid sample from the collection container.
Solid-liquid separation techniques
The samples were collected from 11 pig farms that used seven different kinds of SLS processes. Table 1 shows the sampling site, pig population (head) and the technologies they used while this study was being conducted. The average livestock population was 3,233 heads in surveyed farms and the finishing pig slurry was used for this study. Six out of 12 farms in this study paired screw press with other SLS processes and three farms used coagulation agents. The samples were collected after 5 to 30 minutes of pre-operation based on the characteristics of each solid-liquid separator from individual pig farms. The separated solids were sampled from the solid containers after 30 minutes of solid-liquid separator operation, and then collected solids were mixed uniformly and weighed on site. The influents and separated liquids were collected by installing T-shaped valves on the inlet and outlet. On farms where installing valves was not possible, the influents and treated liquid were collected from the inlet and discharge pipes. The influents and separated liquids were collected when the treated solids were past their half-time. The chemical analysis of the influents, separated liquids, and separated solids consisted of pH, EC, TN, TP, K, TS, SS, NaCl, and heavy metals. The CODMn, BOD5, were analyzed for influent and separated liquid, and the moisture content was only analyzed for separated solids. These analyses were conducted according to the standard method for the examination of water and wastewater. The pH and EC were measured by YSI meter (multilab IDS 4010-2, xylem Inc. US). The heavy metal content (Cu, and Zn) was measured using Spectroblue IPS.OES (FMX36, Germany) based on the US EPA method 7476.
Table 1. The list of sampling sites and their solid–liquid separation (SLS) techniques| Sampling site | Pig (head) | Solid-liquid separation (SLS) | Abbreviations |
| Pig farm A (Nonsan) Pig farm B (Yeoju) | 2,000 2,200 | Drum screen (+ScrewPress) | D/S |
| Pig farm C (Gongju) Pig farm D (Jincheon) Pig farm E (Yeongcheon) | 1,000 6,000 10,000 | Inclined screen (+Screw Press) | I/S |
| Pig farm F (Icheon) | 1,600 | Vibration screen (+ScrewPress) | V/S |
| Pig farm G (Gumi) Pig farm H (Boeun) | 3,000 1,000 | Screw Press | S/P |
| Pig farm I (Hapcheon) Pig farm J (Yeongju) | 2,000 3,800 | Centrifuge | Cf |
| Pig farm I (Hapcheon) Pig farm J (Yeongju) | 2,000 3,800 | Centrifuge (+coagulationagent) | Cf (+Cog) |
| Pig farm K (Changwon) | 4,200 | Belt Press (+coagulationagent) | B/P (+Cog) |
The removal efficiency was calculated using the formula below.
Where, η : removal rate (%)
A. A : Concentration before treatment
B. B : Concentration after treatment
Results and Discussion
Table 2 shows the changes in physicochemical and nutrient parameters by different SLS processes
Table 2. Changes of physicochemical and nutrient parameters (mean value) by different SLS processes (n=3).| Items | Units | stages | +V/S | +D/S | +I/S | +S/P | +Cf | +Cf(+Cog) | +B/P(+Cog) |
| pH | Influents | 6.90 | 7.08 | 7.20 | 7.00 | 7.28 | 7.40 | 7.15 | |
| Separated liquid | 6.65 | 7.23 | 7.23 | 6.88 | 7.25 | 7.53 | 7.20 | ||
| EC (mS/cm) | Influents | 50.10 | 34.88 | 22.77 | 44.63 | 28.08 | 29.85 | 32.65 | |
| Separated liquid | 42.60 | 33.70 | 21.13 | 41.05 | 27.65 | 17.75 | 31.90 | ||
| (Reduction, %) | (15.0) | (3.4) | (7.2) | (8.0) | (1.5) | (40.5) | (2.3) | ||
| NaCl | (%) | Influents | 1.38 | 0.68 | 0.63 | 2.43 | 0.72 | 0.75 | 0.80 |
| Separated liquid | 1.42 | 0.67 | 0.43 | 2.32 | 0.69 | 0.19 | 0.22 | ||
| (Reduction, %) | (-2.3) | (0.5) | (32.0) | (4.7) | (4.9) | (74.6) | (72.9) | ||
| TS | Influents | 15.82 | 5.53 | 3.47 | 12.55 | 4.19 | 4.68 | 8.24 | |
| Separated liquid | 12.17 | 4.82 | 2.78 | 10.74 | 3.39 | 1.50 | 1.75 | ||
| (Reduction, %) | (23.0) | (12.8) | (19.9) | (14.5) | (19.0) | (67.9) | 78.8) | ||
| SS | Influents | 10.42 | 3.99 | 2.30 | 9.42 | 2.74 | 3.21 | 6.43 | |
| Separated liquid | 7.92 | 3.44 | 1.98 | 8.66 | 1.86 | 1.11 | 0.20 | ||
| (Reduction, %) | (24.1) | (13.6) | (14.0) | (8.1) | (32.2) | (65.5) | (96.9) | ||
| TN | (mg/L) | Influents | 14,933 | 7,088 | 3,704 | 10,787 | 5,015 | 5,197 | 7,852 |
| Separated liquid | 12,973 | 6,648 | 3,319 | 10,471 | 4,781 | 3,304 | 4,631 | ||
| (Reduction, %) | (13.1) | (6.2) | (10.4) | (2.9) | (4.7) | (36.4) | (41.0) | ||
| TP | Influents | 4,277 | 1,022 | 793 | 3,285 | 650 | 785 | 1,603 | |
| Separated liquid | 3,874 | 940 | 573 | 2,919 | 457 | 46 | 137 | ||
| (Reduction, %) | (9.4) | (8.0) | (27.7) | (11.2) | (29.8) | (94.2) | (91.5) | ||
| K | Influents | 7,768 | 3,159 | 2,487 | 6,360 | 2,961 | 2,873 | 3,722 | |
| Separated liquid | 7,953 | 3,162 | 2,400 | 6,335 | 2,885 | 2,139 | 1511 | ||
| (Reduction, %) | (-2.4) | (-0.1) | (3.5) | (0.4) | (2.6) | (25.6) | (59.4) | ||
| BOD5 | Influents | 85,350 | 30,290 | 10,229 | 60,585 | 21,948 | 22,825 | 29,580 | |
| Separated liquid | 72,850 | 27,940 | 8,894 | 58,250 | 20,915 | 10,525 | 10,050 | ||
| (Reduction, %) | (14.6) | (7.8) | (13.0) | (3.9) | (4.7) | (53.9) | (66.0) | ||
| CODMn | Influents | 40,527 | 14,923 | 7,822 | 3,0171 | 11,491 | 13,287 | 18,263 | |
| Separated liquid | 34,884 | 14,139 | 6,977 | 2,6291 | 8,295 | 3,245 | 4,104 | ||
| (Reduction, %) | (13.9) | (5.3) | (10.8) | (12.9) | (27.8) | (75.6) | (77.5) | ||
| Zn | Influents | 110.4 | 55.0 | 33.4 | 105.0 | 65.6 | 65.6 | 738.0 | |
| Separated liquid | 104.6 | 50.7 | 28.0 | 109.8 | 57.5 | ND* | 6.8 | ||
| (Reduction, %) | (5.2) | (7.8) | (16.0) | (-4.6) | (12.4) | (100.0) | (99.1) | ||
| Cu | Influents | 66.2 | 19.5 | 16.6 | 56.7 | 24.3 | 24.0 | 47.8 | |
| Separated liquid | 65.9 | 16.8 | 11.0 | 57.3 | 22.1 | 0.3 | 0.9 | ||
| (Reduction, %) | (0.4) | (13.8) | (33.7) | (-1.1) | (9.1) | (98.6) | (98.1) | ||
| +MC | (%) | Influents | - | - | - | - | - | - | - |
| Separated solid | 73.2 | 68.6 | 74.7 | 65.7 | 47.4 | 74.0 | 76.4 | ||
Changes in EC and NaCl content
The different screen mesh sizes from different SLS methods create differences between operation and removal efficiencies of SLS systems and manure characteristics 19). The pH and EC are two important manure characteristics, especially when manure is used for liquid or compost fertilizer. The changes of pH and EC by SLS processes show similarities with the finding of Jørgensen and Jensen (2009) 19), where separated liquids from the influents showed stable pH changes but variable EC changes. Our values show that the pH units remained at 6.61–8.63, which were near neutral, for 44 samples, while EC varied from 2.57 to 4.47 mS/cm. They also show that the Centrifuge (+coagulation agent) had the highest reduction of EC. The near-neutral pH (6.7 to 7.2 units) and widely varied EC (4.9–17.0 mS/cm) are also reflected in studies by Kumaragamage et al. (2016) 21) and Vanotti et al. (2018)14). However, the samples that used coagulation agents showed a little increment in pH that because of the chemical compositions of the coagulant agents but stayed near neutral 11,12,20). In this study, the pH range of influents and separated liquids did not change much by any of the SLS processes (Table 2). The pH changed from 6.90 162 to 6.65, 7.08 to 7.23, 7.20 to 7.23, 7.00 to 6.88, 7.28 to 7.25, 7.40 to 7.53, and 7.15 to 7.20 units for the V/S, D/S, I/S, S/P, Cf, Cf (+Cog), and B/P (+Cog) processes, respectively. The electrical conductivity (EC) reduction by Cf (+Cog) of 40.5% was the highest when the influent EC was 29.8 mS/cm and the separated liquid contained 17.8 mS/cm EC. For the V/S, the influents had the highest EC value, 50.1 mS/cm, but it was reduced 15.0% to 42.6 mS/cm after the treatment. The lowest EC removal was observed for the Cf, where EC reduction was only 1.5%, from 28.1 mS/cm to 27.7 mS/cm. On the contrary, B/P (+Cog) with a coagulant agent did not show much EC reduction (2.3%). The NaCl reduction by Cf (+Cog) and B/P (+Cog) with a coagulation agent showed the highest removal efficiency at 74.6% and 72.9%, respectively (Table 2). Among the three screening separator processes, the V/S added up to 2.3% NaCl, I/S reduced 32.0%, and D/S reduced 173 only 0.5%.
Changes of TS and SS contents
Figure 1 shows the changes in TS and SS by different SLS processes. The B/P (+Cog) and the Cf (+Cog) had 78.76% and 67.94% TS removal efficiency, respectively, the highest. Excepting these two coagulations agents–supported SLS processes, the rest of the SLS processes showed 12.8~23.0% TS removal efficiency. The coagulant-supported SLS processes had the highest SS reduction: 96.9% and 67.94% by B/P (+Cog) and Cf (+Cog), respectively. The Cf process had SS removal efficiency of 32.2%. The rest of SLS processes showed 8.1~24.1% TS removal efficiency. Meanwhile, the moisture in the separated solids was 73.2%, 68.6%, 74.7%, 65.7%, 74.0%, and 76.4% for the V/S, D/S, I/S, S/P, Cf (+Cog), and B/P (+Cog) processes, respectively. However, the Cf process contained the lowest moisture content at 47.41%.
Figure 1.Changes in TS (A) and SS (B) by different SLS processes
Changes in nutrient content (TN, TP, and K), BOD5, and CODMn
Comparing the screw press (S/P) with a centrifuge (Cf), several authors found that the centrifuge achieves higher performance 8,10,22). According to Aguirre-Villegas et al (2019) 14), Cf processes are more effective for the removal of TS, SS, TN, TP, K, BOD5, and CODMn than S/P processes. This finding is also reflected in this study (Table 1). Figure 2 shows the changes in nutrient content (TN, TP, and K), BOD5, and CODMn by different SLS processes. When comparing Cf process with V/S, D/S, V/S shows better removal of TS, TN, and BOD5. The Cf (+Cog) process had a better EC, NaCl, TS, and TP removal 9 efficiency than the B/P process. On the other side, the B/P (+Cog) process had a comparatively better performance for TN, K, BOD5, and CODMn removal than the Cf (+Cog) process. The relatively low removal efficiency of TN, TP, and K in separated liquids by mechanical processes without coagulation agents could be because of their higher suable or dissolved form 12,14,23,24). The higher TP concentrations in separated liquids and the removal efficiency for B/P (+Cog) are similar to those in a study conducted by Møller et al. (2002) 25). However, the higher removal efficiency of TN, TP, and TK by Cf (+Cog) and B/P (+Cog) is due to the chemical treatment coupled with mechanical (centrifuge and belt press) treatment, which screened out smaller sized particles 20,26). The B/P (+Cog) showed the highest TN reduction of 41.0%, where the influents had 7,857 mg/l, and the separated liquid had 4,631 mg/l of TN. The Cf (+Cog) had the second highest TN removal of 36.4%, where the TN reduced from 5,197mg/l to 3,304mg/l. However, V/S, D/S, I/S, S/P, and Cf showed 2.9~13.1% TN removal efficiency. The removal of TP had similar results as that of TN. The Cf (+Cog) and B/P (+Cog) processes showed 94.2% and 91.5% of TP removal efficiency, respectively, in separated liquids. However, V/S, D/S, I/S, S/P, and Cf showed 8.0~29.8% TN removal efficiency. The B/P (+Cog) and Cf (+Cog) showed 59.4% and 25.6% K removal efficiency, respectively. The I/S, S/P, and Cf had only 0.4~3.5% removal efficiency for K. Moreover, in the case of V/S and D/S, the concentration of K somewhat increased somewhat in separated liquids. The B/P (+Cog) and Cf (+Cog) showed 59.4% and 25.6% K removal efficiency, respectively. The I/S, S/P, and Cf had only 0.4~3.5% removal efficiency for K. Moreover, in the case of V/S and D/S, the concentration of K somewhat increased in separated liquids. The concentration of CODMn and BOD5 indicates the presence of oxygen-demanding substances in wastewater and are often used as pollution indicators. In this study, the chemical coagulant agent–supported centrifugal and belt press SLS were able to remove the highest amount of BOD5 and CODMn. The B/P (+Cog) reduced 66.0% of BOD5, and the Cf (+Cog) reduced 53.9% of it, and the concentration of BOD5 in influents was 29,580 mg/l and 22,825 mg/l, and after treatment in separated liquid that reduced to 10,050 mg/l and 10,525 mg/l, respectively. The removal of CODMn had similar results as that of BOD5, comparatively. The Cf (+Cog) and B/P (+Cog) processes showed 75.6% and 77.5% of CODMn removal efficiency, respectively, in separated liquids. However, other SLS processes showed low removal efficiency for BOD5 (4.7~14.6%) and CODMn (5.3~27.8%). About 40% of BOD5 was found in a solid fraction of manure; therefore, after performing SLS, about 60% of the BOD5 was supposed to remain in the separated liquid 27). Therefore, the TS and BOD5 10 concentration in separated liquids remained in the same sequential order of V/S, S/P, D/S, Cf, I/S. For the coagulation agent–assisted SLS processes, the respective TS and BOD5 removal was 67.9% and 53.9% for Cf (+Cog) and 78.8% and 66.0% for B/P (+Cog). Similar properties were noticed in changes in CODMn. Each sample that contained higher TS concentrations in both the influent and separated liquids showed higher CODMn concentrations as 28).
Figure 2.Changes in TN (A), TP (B), K (C), BOD5, and CODMn (D) by different SLS 245 processes
Changes in Zn and Cu
Separating of heavy metals such as Zn and Cu from liquid manure before land application reduces the risk of soil contamination 14,29). Among all the analyzed heavy metal contents, Zn and Cu were found in a higher concentration in the influents. The probable cause is that the pigs get the Zn and Cu from their feeding operation 30). Figure 3 shows the changes in nutrient content Zn and Cu by different SLS processes. The coagulant assist centrifuge (Cf (+Cog)) and belt press (B/P (+Cog)) were very effective at reducing the heavy metals such as Zn (100% and 98.6%) and Cu (99.1% and 98.1%). These findings are similar to those of Vanotti et al. (2018)11), which found that a PAM-based mechanical pressing SLS system could remove 88% of Zn and Cu. However, contrastingly, other SLS processes showed low removal efficiency for Cu and Zn. The V/S, D/S, I/S, and Cf showed 5.2~12.4% Zn removal efficiency and 0.4~33.7% Cu removal efficiency, respectively. Moreover, in the case of the I/P process, the concentration of Zn and Cu somewhat increased in separated liquids.
Figure 3.Changes in Zn (A) and Cu (B) by different SLS processes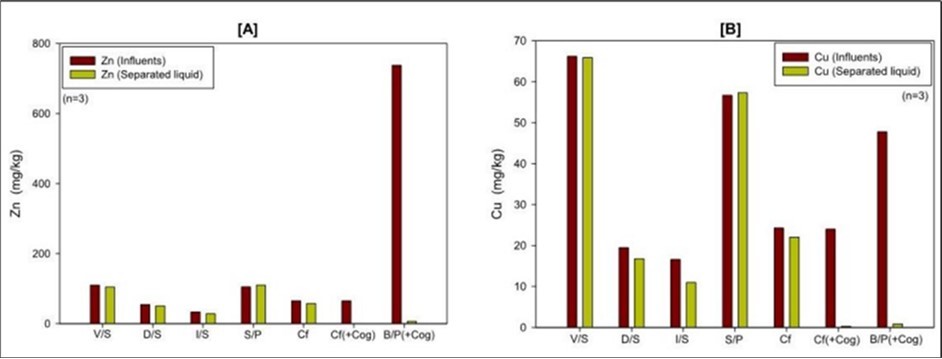
Conclusions
This study has assessed the separation and/or reduction efficiency of various SLS technologies used in pig farms. The centrifugal and belt press processes that used chemical coagulation showed much more removal of nutrient contents, solids, and metals like Zn and Cu. Among all the SLS processes studied, the Cf (+Cog) process showed a 40.5% EC reduction, the highest among all SLS processes. The NaCl was reduced by the Cf (+Cog) and B/P (+Cog) by 74.6% and 72.9%, respectively. The B/P (+Cog) process had the highest TS and SS reduction at 78.8% and 96.9%, respectively. The highest TN and TP removals were 41.0% and 94.2% by B/P (+Cog) and Cf (+Cog), respectively. The B/P (+Cog) removed 59.4% and 66.0% of BOD5 and CODMn, respectively. The Zn and Cu were reduced by 100% and 98.6% by Cf (+Cog), respectively. However, V/S, D/S, I/S, S/P, and Cf showed lower removal efficiency of nutrient contents, solids, Zn, and Cu than centrifugal and belt press processes that used chemical coagulation. In this study, we found that chemical coagulants made a notable difference in SLS performance for the removal of or changes to different physiochemical parameters of pig manure. Although SLS is an effective pre-treatment process for liquid manure treatment and using chemical coagulants helps remove excess fine solids, nutrients, and heavy metals, further studies are needed to determine how coagulants agents react with other SLS and liquid manure 12 treatment processes.
Authors and Contribution Statement
Sooryang Kim
Industry-Academic Cooperation Foundation Sangji University Republic of Korea, Research Professor,ORPID(0000-0001-6449-5100), Data analysis, Writing – original draft, Writing – review and editing
Seunghyun Park
Department of Environmental Engineering Sangji University Republic of Korea, M.D. Student, ORCID(0009-0000-8845-2295), Writing – original draft, Writing – review and editing
Suchan Lee
Department of Environmental Engineering Sangji University Republic of Korea, M.D. Student, ORCID(0009-0007-6320-1407), Writing – original draft, Writing – review and editing
Jiwon Jung
Department of Environmental Engineering Sangji University Republic of Korea, M.D. Student, ORCID(0000-0002-4474-1105), Writing – original draft, Writing – review and editing
Sanghyean Ahn
Department of Environmental Engineering Sangji University Republic of Korea, M.D. Student, ORCID(0009-0001-7059-0312), Writing – original draft, Writing – review and editing
Kwonwoong Ham
Department of Environmental Engineering Sangji University Republic of Korea, M.D. Student, ORCID(0009-0008-6730-5972), Writing – original draft, Writing – review and editing
Myunggyu Lee
Department of Smart life science Sangji University Republic of Korea, Professor, 16 ORCID(0000-0002-8014-5490), Project administration, Supervision
Acknowledgements
This work was supported by by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture and Forestry (IPET) and the Korea Smart Farm R&D Foundation (KosFarm) through the Smart Farm Innovation Technology Development Program, funded by the Ministry of Agriculture, Food and Rural Affairs (MAFRA), Ministry of Science and ICT (MSIT), Rural Development Administration (RDA) (421046-03), and the Sangji University of Graduate School.
References
- 1.A F Bouwman, Booij H. (1998) Global use and trade of foodstuffs and consequences for the nitrogen cycle. , Nutr. Cycl. Agroecosys 52, 261-267.
- 2.H C Le. (1998) Biodigester effluent versus manure, from pigs or cattle, as fertilizer for duckweed (Lemna spp.). , Livest. Res. Rural Dev 10(3), 56-65.
- 3.Hjorth M, K V Christensen, M L Christensen, S G. (2010) Solid-liquid separation of animal slurry in theory and practice. A review. , Agron. Sustain. Dev 30, 153-180.
- 4.C H Burton, Turner C. (2003) . Manure Management: Treatment Strategies for Sustainable Agriculture, 2nd ed., Silsoe Research Institute, UK. 4 .
- 5.Steinfeld H, Gerber P, Wassenaar T, Castel V, Rosales M et al. (2006) Livestock’s Long Shadow: Environmental Issues and Options. , FAO, Rome, ISBN 978, 92-5.
- 6.M P Bernal, Roig A, Madrid R, A F Navarro. (1992) Salinity risks on calcareous soils following pig slurry applications. , Soil UseManage 8, 125-130.
- 7.M P Bernal, Roig A, García D. (1993) Nutrient balances in calcareous soils after application of different rates of pig slurry. , Soil Use Manage 9, 9-14.
- 8.H B Møller, J D Hansen, C A G. (2006) Sørensen. , American Society of Agricultural and Biological Engineers 50(1), 193-200.
- 9.M R Teira-Esmatges, Flotats X. (2003) A method for livestock waste management planning in NE Spain. , Waste Management 23, 917-932.
- 10.H B Møller, Lund I, S G. (2000) Solid-liquid separation of livestock slurry: 13 Efficiency and cost. , Bioresource Tech 74(3), 223-229.
- 11.M B Vanotti, K S Ro, A, J H Loughrin, P D Millner. (2018) . High-Rate Solid-Liquid Separation Coupled With Nitrogen and Phosphorus Treatment of Swine Manure: Effect on Water Quality. Frontiers in Sustainable Food Systems 2(49), 1-15.
- 12.H A Aguirre-Villegas, R A Larson, A M.Sharara 2019 Anaerobic digestion, solid liquid separation, and drying of dairy manure: Measuring constituents and modeling emission. , Science of the Total Environment 696, 134059-11.
- 13.J P Chastain, M B Vanotti, M. (2001) Effectiveness of liquid-solid separation for treatment of flushed dairy manure: a case study. , Appl. Engr. Agric 17, 343-354.
- 14.Riaño B, C M.García-González 2014 On-farm treatment of swine manure based on solid-liquid separation and biological nitrification-denitrification of the liquid fraction. , Journal of Environmental Management 132, 87-93.
- 15.K B Cantrell, Ducey T, K S Ro, P G Hunt. (2008) Livestock waste-to-bioenergy generation opportunities. , Bioresource Technology 99, 7941-7953.
- 16.Steinmetz R L R, Kunz A, V L Dressler, Flores E M M, F A. (2009) Martins. , CLEAN: Soil, Air, Water 37, 239-244.
- 17.Kumaragamage D, O, Greiger L. (2013) Phosphorus fractions in solid and liquid separates of swine slurry separated using different technologies. , J. Environ. Qual 42, 1863-1871.
- 18. (2008) Korean Ministry of Environment (KMOE). , KMOE, Waste-to-Energy Division, Sejong, South Korea 18.
- 19.Kunz A, Steinmetz R L R, M, Coldebella A. (2008) Effect of storage time on swine manure solid separation efficiency by screening. , Bioresource Technology 100, 5485-5489.
- 20.Jørgensen K, L S Jensen. (2009) Chemical and biochemical variation in animal manure solids separated using different commercial separation technologies. , Bioresource Technology 100, 3088-3096.
- 21.Kumaragamage D, O, G J Racz. (2016) Comparison of Nutrient and Metal Loadings with the Application of Swine Manure Slurries and Their Liquid Separates to Soils. , J. Environ. Qual 45, 1769-1775.
- 22.C A Gooch, S F Inglis, Czymmek K. (2005) Mechanical solid-liquid manure separation: performance evaluation on four New York state dairy farms - a preliminary report. Annual International Meeting. Paper Number: 05–4104. American Society of Agricultural and Biological Engineers , Michigan .
- 23.Bernet N, Béline F. (2009) Challenges and innovations on biological treatment of livestock effluents. , Bioresour. Technol 100, 5431-5436.
- 24.Saeys W, A M Mouazen, Ramon H. (2005) Potential for onsite and online analysis of pig manure using visible and near infrared reflectance spectroscopy. , Biosystems Eng 91, 393-402.
- 26.M B Vanotti, Rashash D M C, P G Hunt. (2002) Liquid–solids separation of flushed swine manure with PAM: effect of wastewater strength. , Trans. ASAE 45, 26.
- 27.Zhu J, P M Ndegwa, Luo A. (2001) Effect of solid–liquid separation on BOD and VFAs in swine manure. , Environ. Technol 22, 1237-1243.
- 28.J P Chastain, M B Vanotti. (2003) Correlation Equations to Predict the Solids and Plant Nutrient Removal Efficiencies for Gravity Settling of Swine Manure. In:. R.T. Burnes (ed), Animal, Agricultural and Food Processing Wastes IX : Proceedings of the Nineth International Symposium, Research , Triangle Park, NC 487-495.
- 29.Bolan N, Adriano D, Mahimairaja S. (2004) Distribution and bioavailability of trace elements in livestock and poultry manure by products. , Crit. Rev. Environ. Sci. Technol 34, 291-338.
- 30.Zhang F, Li Y, Yang M, Li W. Suchan Lee Department of Environmental Engineering Sangji University Republic of Korea (2012) Content of heavy metals in animal feeds and manures from farms of different scales in northeast. Writing – original draft, Writing – review and editing Sanghyean Ahn Department of Environmental Engineering Sangji University Republic of Korea, M.D. Student, ORCID(0009-0001-7059-0312), Writing – original draft, Writing – review and editing Kwonwoong Project administration, Supervision , Writing – original draft, Writing – review and editing Jiwon Jung 16, 0009-0000.

