Camel Brucellosis in Ethiopia: Seroprevalence and Associated Risk Factor
Abstract
Camels are a significant source of income for nomadic populations in many developing countries, including Ethiopia. Camels are well adapted to dry and semi-dry regions, providing income, food security, and transportation. However, camel production and productivity are constrained by infectious diseases, such as brucellosis, which is a highly infectious bacterial disease that affects camels and humans worldwide. Brucellosis causes significant economic losses due to abortion, low herd fertility, and decreased milk production. In Ethiopia, the prevalence of camel brucellosis varies depending on factors related to the host, agent, climate, and management system, with a reported prevalence ranging from 0.5% to 11.9%. Accurate diagnosis of camel Brucellosis is essential for herd-based screening of animals. Although culturing the pathogen is the preferred method for diagnosis, serological tests such as Rose-Bengal plate test (RBPT), Enzyme-linked immunosorbent assay (ELISA), and Complement fixation test (CFT) and polymerase chain reaction (PCR) assays have been developed. Implementing effective diagnosis and surveillance systems to control the spread of brucellosis in animals and humans is very important, on top of awareness campaigns, vaccination programs, and suitable laboratory establishment recommended. Continued research is essential to maintain the health and productivity of camel populations, particularly in pastoral areas where camels play a significant role in the livelihood of communities. Therefore, the present paper views the seropositive prevalence and potential risk factors associated with camel brucellosis in Ethiopia.
Author Contributions
Academic Editor: Mohammed A Elmetwally, Professor of Theriogenogy
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2023 Wario Waji, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Camels are part of the Camelidae family and play a crucial socio-economic role in dry and semi-dry regions worldwide1. They are used for income, food security, and transportation. In Ethiopia, camels are a significant subset of livestock, with a population of approximately 2.3 million, ranking third in Africa and fourth in the world2. Camels are well adapted to the harsh environmental conditions in arid and semi-arid areas, making them versatile and vital domestic animals. In Ethiopia, they are important livestock in pastoral areas, and the expansion of dromedary camels in East African countries like Ethiopia is influenced by ecological changes, socio-cultural conditions, and recurring droughts3.
Dromedaries are highly drought tolerant and thrive in arid countries where other domesticated animals cannot survive. They can graze on low-productive pastures and produce a high amount of milk due to their ability to feed on plants that other animals cannot4. Camels are important for ensuring food security in pastoral communities. However, camel production and productivity are constrained by factors such as infectious diseases, including brucellosis, which can cause stillbirth, mastitis, decreased milk production, and reproductive failure in animals5, 6.
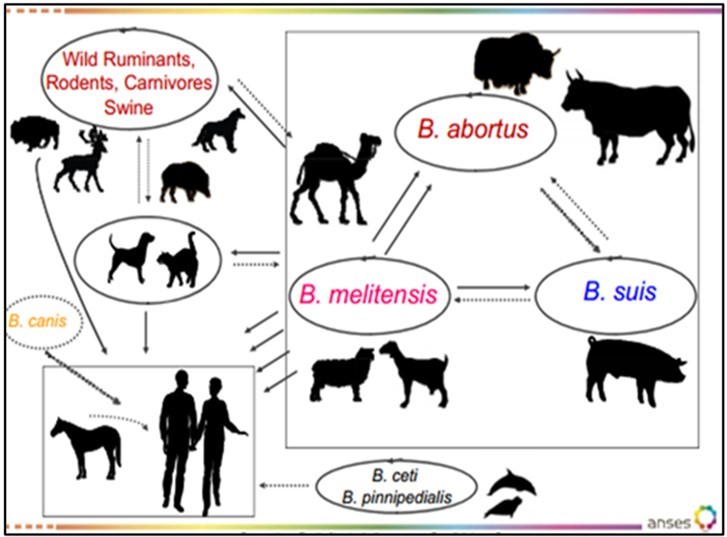
Camel Brucellosis causes economic losses due to infertility, abortions, mastitis, delayed first calving, prolonged calving intervals, and decreased milk production. Infertility is characterized by increased inter-calving period and abortion results in loss of neonatal calves7, 8. Brucella abortus (B. abortus), Brucella melitensis (B. melitensis) and Brucella ovis (B. ovis) mainly cause camel brucellosis9. B. abortus and B. melitensis are the most frequently isolated Brucella species from milk, aborted fetus, and vaginal swabs of diseased camels. Camels are susceptible to both B. abortus and B. melitensis. The prevalence of Brucella in camels depends on the infection rate in primary hosts that encounter them. The bacteria can enter the body of animals through inhalation, ingestion, or through mucous membranes or broken skin. Inflammation can occur in different organs, causing orchitis and epididymitis in males and placentitis, abortion, and infertility in females10. Camels frequently contract brucellosis from infected ruminants, and outbreaks with classical signs have been described. The disease is rare in camels not in contact with ruminants, but it remains a concern in pastoral areas due to lack of awareness and consumption of raw milk11.
Brucellosis is highly contagious, zoonotic, and economically important worldwide12. Humans can acquire the disease through physical contact with infected livestock and consumption of raw milk13. Brucellosis remains widespread in domesticated and wild animal populations, presenting a great economic problem for tropical animal husbandry and is one of the most economically important diseases in developing countries14.
The isolation and identification of the disease from the animals' aborted materials, udder secretions or from tissues removed at post-mortem or patient’s serum by detection of specific antibodies using appropriate serological methods. A presumptive diagnosis can be made by assessing specific cell-mediated or serological responses to Brucella antigens. All Brucella are related to lifelong chronic animal infection since they are found within the cells of their milk glands and reproductive system. In Ethiopia, the eastern and southern parts of Ethiopia, namely, Afar, Somali and Borena are the major areas where camel husbandry is widely practiced insuring the livelihood of the pastoral communities. In these regions and others, brucellosis in animals and humans has been reported where the prevalence was quite varying under different agroecology. Consequently, this disease has resulted in significant economic and public health problem in the stated area. So to effectively control camel brucellosis is paramount important to establish diagnostic and surveillance systems, by estimating the cost-benefits of control measures 15, 16.
The serological tests like RBPT are accurate diagnosis of camel brucellosis, it is also cheap and easy for herd-based screening of animals with high sensitivity and low specificity, whereas tests like ELISA and CFT are used for a confirmatory test. Generally, despite the presence of large population of the camel in the pastoral areas of Ethiopia (16, 17, reports of camel brucellosis and studies of management practices are limited. Additionally, even if the disease is one of the oldest recognized diseases of mankind and get controlled in most developed countries 14. Concomitant to Camel brucellosis, the major animal disease prevalence in Ethiopia is tightly associated with poor animal health care and extension service, lack of awareness by the farmers on how to contain the above prevailing constraints18, 19. Only little effort has been made to control this disease in developing countries, especially in Ethiopia due to the nature of diseases. The objective of this review seminar paper is to compile the seroprevalence study and potential risk factors associated with Camel brucellosis in Ethiopia.
General Review on Camel Brucellosis
Etiology
Brucella, a genus of bacteria named after David Bruce, are small, coccobacilli shaped rods measuring 0.5 x 0.7 to 0.6 x 1.5μm. They occur either singly, in pairs or in short chains, and are non-spore-forming, non-motile, partially acid-fast and Gram-negative facultative intracellular bacteria. While most strains are aerobic and some are micro-aerophilic, many of them are capnophilic and require a CO2-enriched atmosphere for optimal growth. Certain strains are also carboxyphilic and require a 5% to 10% carbon dioxide atmosphere for growth, while others grow aerobically. Brucella causes a contagious disease, and its subdivision is determined based on biochemical reactions and agglutination with mono-specific sera20. table 1
Table 1. Summarizes Brucella strains, hosts and its mode of transmission.| Strain Symptoms | Host | Other Hosts | Symptoms | Transmission | Human Disease |
|---|---|---|---|---|---|
| B . abortus | Cattle | Sheep, dogs, goats, pigs, horses, humans, wild ungulates | Abortion after 5 months | Ingestion, some venereal | undulant fever-control with antibiotics |
| B. melitensis | Sheep goats | buffalocattle, pigs, dogs, humans, camels | Later term abortion weak young, mastitis (goats) | Ingestion | Malta fever: can be fatal in human |
| B . ovis | Sheep | most often effects rams, | rare abortions | ||
| B . suis | Pig | cattle, horses, dogs, human reindeer, caribou | Abortion and infertility | Ingestion and venereal extremely | deadly in human |
| B . c ani s | Dogs | Humans | abortions at 40-60 days | Venereal | mild disease in humans |
Common Characteristic of Camel Brucellosis
Brucella are Gram-negative bacteria that are capnophilic, aerobic, and facultative intracellular parasites. They are coccobacillary in shape, non-motile, and non-spore-forming. Brucella do not grow on MacConkey agar, but they are urease-positive, with the exception of B. ovis 25. All Brucella strains are catalase and oxidase-positive, except for B. ovis and B. abortus biotype 2. Their growth is enhanced by blood serum on basal medium. Brucella species commonly inhabit the ungulate placenta and fetal tissue in females, as well as testis fluid in males26.
Mode of Transmission of Camel Brucellosis
Animal brucellosis can be transmitted both vertically and horizontally. Horizontal transmission occurs through ingestion, inhalation, wounds, intact skin, conjunctiva, and venereal contact, and is a major route of infection for many species of Brucella. Venereal transmission is the primary mode of transmission for B. ovis. While most Brucella species are rarely transmitted through natural mating, artificial insemination using infected semen is a significant route of infection for animals. The organism is transported to the thoracic duct via lymphatic channels and then disseminates to parenchymal and joint tissues through the bloodstream 27. The mode of transmission of Brucellosis is depicted in Figure 1 below.
Diagnosis and Treatment of Camel Brucella
The diagnosis of Brucella is confirmed by different methods, according to the availability of appropriate medium, serological test and molecular technique.
Clinical signs
Brucellosis causes abortion, stillborn calves, retained placenta, fetal death, mummification, and reduced milk yield in female camels. In males, it can cause orchitis and infection of the accessory sex glands. Clinical signs in breeding camelids are similar to those in bovines and small ruminants, but with fewer abortions reported29. Retained placenta is rare in Camelidae due to differences in placental attachment. Necropsy findings include focal granulomas in the liver and generalized lymphadenitis or supramammary lymph node, with the pathogen reisolated from the lymph nodes of the genital tract and head30.
Pathology
Brucella organisms cause various pathological alterations in camelids, affecting tissues such as the pregnant uterus, udder, testicles, accessory male sex glands, lymph nodes, joint capsules, and bursae31. Inflammation, oedema, and necrotic foci in the uterus epithelium, fibrosis of the endometrium, and atrophy of the uterine glands are common. Bacteria were found in multiple organs and mammary gland lymph nodes. Lesions were also observed in lactating dromedaries seropositive for B. melitensis, and B. abortus was isolated from milk samples. Histological findings showed sub-acute placentitis and capillaries expanded by Brucella organisms. Non-pregnant dromedaries showed only a few lesions32.
Bacteriological Diagnosis
For bacteriological diagnosis in the laboratory, there are procedures to identify and characterize Bacteria morphology depend on typical characteristics through primary and secondary identification and serology test. The morphology of the Brucella colonies is associated with the presence of lipopolysaccharides (LPS) in the external membrane of the bacterium. Smooth (S-LPS) and rough (R-LPS) phenotypes are differentiated. The S-LPS phenotype is found in most Brucella species. Some proteins of Brucella are responsible for serological cross-reactions between Brucella and other bacterial species 26.
Due to the resemblance of Brucella to other gram-negative bacteria, it can be challenging to identify them with a single test. However, direct demonstration of the causal organism using staining, immunofluorescent antibody, culture, and serological techniques can help in diagnosis33. The selection of samples for culture depends on the clinical signs observed, and the preferred tissues include those of the reticuloendothelial system, pregnant uterus, and udder. Growth can be observed after 3-4 days, but negative cultures should not be discarded until 7-10 days have elapsed34. Specimens can be stained using Gram stain and modified Ziehl-Neelsen stains to observe small, red-colored coccobacilli in clumps. To check for bacterial and fungal contamination, Brucella selective media can be used, which are nutritive blood agar-based with 5% seronegative equine or bovine serum. Guinea pigs are the most sensitive laboratory animals for the isolation of Brucella species, particularly from internal organs like lymph glands, testes, and vagina33.
Culture
The laboratory diagnosis of brucellosis typically involves the culture of blood, milk, or tissue, as well as uterus discharge. Brucella organisms can be retrieved from the placenta, but it is more convenient to obtain them in pure culture from the stomach and lungs of aborted fetuses. Farrell’s medium, which contains six antibiotics, is the recommended medium for isolation. Other selective Brucella media are also available for the growth of this pathogen from fresh camel milk and camel tissue samples35. Given the high number of cases, it is preferable to use selective media. Tissue specimens from Brucella-positive dromedaries were examined using the immunoperoxidase test, yielding very good results. Brucella organisms were detected in the cytoplasm of macrophages (visible as brown granules), in the lymphocytes of the lymph nodes and spleen, within the epithelial lining of the endometrium and endothelium of blood vessels, and within mononuclear cells around blood vessels36.
Molecular diagnosis
Molecular diagnosis is the most preferable technique for diagnosing brucellosis at the genetic level. Polymerase Chain Reaction (PCR) is a new approach that has been developed to overcome limitations and difficulties in bacterial culture and serological assays. PCR-based assays are based on the detection of specific gene sequences of the pathogens, and allow typing of the isolated strains. Although the isolation of Brucella organisms is still the preferred method of diagnosis, PCR shows high sensitivity and specificity. One of the first PCR assays to differentiate among Brucella species was called AMOS-PCR. New PCR techniques are being implemented for both identification and phenotypic biotyping, including multiple Locus Variable Number tandem repeat analysis37.
Serology
Rose Bengal Plate Test (RBPT)
The RBPT is a primary, rapid, and simple slide-type agglutination assay used for serum serological testing. It has a high sensitivity (>99%) but disappointingly low specificity (68.8%) when screening for antibodies against Brucella38. The principle behind the test is the agglutination of serum antibodies with Rose Bengal dye-stained B. abortus whole cells buffered at a pH of 3.65. Despite its low specificity, it is still commonly used for brucellosis screening purposes in resource-limited laboratories and high-risk rural areas where other tests may not be possible39.
Indirect Enzyme-Linked ImmunoSorbent Assay (I-ELISA)
ELISA test is a secondary serological test that high sensitivity and specificity than RBPT. The samples that were screened positive by RBPT were further confirmed by I-ELISA for the detection of Brucella antibodies. I-ELISA seems to be an important alternative to the conventional serodiagnosis of camelid brucellosis. I-ELISA is used to discriminate between the presence of specific IgM and IgG antibodies and to roughly access the stage of illness 40.
Complement fixation test (CFT)
The test that can be used to detect the presence of either a specific antibody or specific antigen in the serum. It was widely used to diagnose, particularly with a microbe that is not easily detected by culture method. All sera reacted positive to the RBPT were further tested using CFT for confirmation. The control sera and complement were both obtained from the Federal Institute for Health Protection of Consumers and Veterinary Medicine, Germany standard B. abortus antigen for CFT (Veterinary Laboratories Agency, United Kingdom) was employed to detect the presence of antibodies against Brucella in the sera. The CFT measures more antibodies of the IgG1 than antibodies of the IgM type, Since it usually appears after antibodies of the IgM type, control and surveillance for brucellosis is best done by CFT 41.
Treatment of Camel Brucellosis
Antibiotics; the bacteria is gram-negative facultative intracellular parasite and morphologically coccobacilli which are sensitive to many broad-spectrum antibiotics, but the use of antibiotics is forbidden in many countries because of the uncertainty related to the infective status of the treated animals and because of the spread of antibiotic resistance. Treatment is unlikely to be cost-efficient or therapeutically effective because of the intracellular sequestration of the organisms, mainly in the lymph nodes. In addition to this treatment, milking camels received 10 ml of ox tetracycline as intramammary infusions in each teat every two days for eight days. All treated dromedaries also became serologically negative within 16 months of treatment. This regimen of treatment was effective in eliminating the shedding of Brucella organisms through milk. But the single untreated control camel remained positive over the same period of time 42.
Seroprevalence and Associated Risk Factor of Brucellosis
Status of Brucella in Ethiopia
Ethiopia has a high population of camels and is known for its low-lying pastoral areas, which are particularly suitable for camel breeding and other livestock. These lowlands are predominantly located in the Eastern, South-Eastern, and Southern regions of the country, accounting for 40% of the livestock population 43. The traditional practice of mixing camels with other animals in an extensive system has led to inadequate care during watering and migrations. The prevalence of Brucella infection is heavily influenced by management practices and climate conditions. Despite the abundant livestock resources scattered throughout Ethiopia's diverse agro-ecologies, they remain underutilized 44. Ethiopia's agro-ecologies can be broadly classified as highlands and lowlands 45.
Previous research has reported on the prevalence of camel brucellosis in Ethiopia, with rates ranging from 0.73% to 11.9% for RBPT and 0.53% to 9.6% for CFT in pastoral areas. These variations in prevalence are believed to be due to differences in animal husbandry and management practices among the pastoralist society46. The consumption of raw camel milk and close contact with animals during abortion and calving without protective measures contributes to the transmission of brucellosis among humans and animals. Unfortunately, over 75% of animal owners are unaware of the risks of zoonotic camel brucellosis, and over three-quarters of pastoralists engage in at least one activity that increases the risk of transmission47. There has been limited research on camel brucellosis in Ethiopia, with initial studies reporting seroprevalence rates of 4.4% and 5.7% using RBPT and CFT in various provinces and regions48. More recent studies conducted in the Borena lowland reported seroprevalence rates of 1.8% using RBPT and 2.2% using CFT, although there were variations in results that could be due to differences in climate, management systems, or test sensitivity/specificity49.
In southeast lowland areas of the Somali Region, Birhanu 50 found a seroprevalence of 2.43% (n=822) in individual camels and a seroprevalence of 10.3% (n=185) in camel herds. Mohamed et al. 51 reported that brucellosis seroprevalence in camels in eastern Ethiopia kept without ruminants, with small ruminants, and with large ruminants was 1%, 4.3%, and 5.3%, respectively. Based on these findings, it can be estimated that keeping camels with small or large ruminants can increase disease prevalence, while keeping camels alone leads to lower disease prevalence. Various researchers have reported different prevalence rates in Ethiopia's different regions and zones. For example, Sisay and Mekonnen 52 reported an RBPT prevalence of 11.9% and a CFT prevalence of 7.6% in the Afar region, while Gumi et al. 53 reported a 3.42 RBPT and 2.43 CFT prevalence in the Somali and Oromia regions. Furthermore, Gessese et al. 54 reported a 0.73% RBPT and 0.53% CFT prevalence in Bale and Borena at export (Table 2).
The variable result reported from different region and zone indicates the prevalence of disease is not uniform, maybe due to lack of awareness of owner to separate infected animals from normal, to vaccinate whole animals, to keep the camel with small or large ruminant, to introduce another animal in herd or due to researcher itself by making fault during sampling, means insufficient sample or careless transport sample or specific and sensitivity of technique or unavailability of reagent and material and inadequate training on how to collect sample and how to interprete the result. The prevalence of brucellosis in Ethiopia is summarized in Table 2 below.
Table 2. Seroprevalence of camel brucellosis in Ethiopia| District | No of animal | Sample taken | Test employed | Prevalence | References |
|---|---|---|---|---|---|
| Afar | 768 | Serum | RBPTCFT | 11.9%(RBPT) 7.6%(CFT ) | 52 |
| 460 | Serum | RBPTCFT | 5.4% | 55 | |
| 1152 | Serum | RBPTCFT | 5.0%(RBPT) 4.1%(CFT) | 16 | |
| 813 | Serum | RBPTCFT | 2.09% | 56 | |
| Somali, afar, oromia | 1442 | Serum | RBPTCFT | 5.7%(RBPT) 4.2%(CFT) | 48 |
| Southern Ethiopia | 1830 | Serum | RBPTELISA | 0.9% | 53 |
| Akaki | 201 | Serum | RBPTCFT | 6.5%(RBPT) 4.5%(CFT) | 11 |
| Jijjiga and Babile | 822 | Serum | RBPT | 2.43% | 17 |
| Dire dawa | 646 | SerumCFT | RBPT | 2% (RBPT)1.5%(CFT) | 57 |
| Borana | 7561073 | Serum Serum | RBPTRBPTCFT | 2.2%1.8% | 49 58 |
| Bale and Borana | 1500 | Serum | RBPT | 0.53% | 54 |
| Yabello | 384 | Serum | RBPTCFT | 3.6%3.1% | 59 |
Risk Factor Associated with Camel Brucellosis in Ethiopia
In areas where wildlife and camel rearing coexist, Brucella species pose a significant risk for the perpetuation of the pathogen within the animal population. Infections in wildlife can also impede eradication efforts in camels. B. abortus remains a human pathogen, and outbreaks resulting from infected camels or consumption of contaminated dairy products present a notable risk of infection. B. melitensis is the primary causative agent of brucellosis in camels and small ruminants, although B. ovis can also infect sheep. Although rare, sporadic cases of brucellosis have been reported in sheep and goats. Both B. abortus and B. melitensis are responsible for camel brucellosis, which is a significant cause of economic loss due to abortions60.
Risk factors associated with Management
According to Radostits et al. 61, unrestricted movement of infected animals from an infected herd to a non-infected herd is the primary mode of Brucella pathogen transmission between herds and areas. Effective management practices can contribute to the prevention of transmission. Large herd size, active abortions, and loose housing can increase the time required for recovery from brucellosis, as noted by Al-Majali et al. 62.
Poor management practices during calving are a significant factor in the spread of brucellosis. Mixing calving pens increases exposure, whereas separate calving pens can reduce exposure of infected animals. Higher population density (number of animals per land area) is positively associated with disease prevalence due to increased contact between susceptible and infected animals. Eliminating infected males and minimizing exposure to aborted tissue through effective management practices can greatly reduce disease incidence. Both direct and indirect exposure to aborted fetuses are critical in maintaining infections in a herd. Introducing infected animals and keeping camels with other ruminants can accelerate the spread of infection within a herd, as noted by Menachem 63.
Risk factors associated with the host.
Age: Traditionally, brucellosis has been viewed as a disease affecting adult animals, as susceptibility to the disease increases after sexual maturity and pregnancy. All breeding male and female camels above six months of age were included in the study. Although infection can occur in animals of all age groups, it is more commonly found in sexually matured animals. Younger animals tend to be more resistant to infection and frequently clear the infection, although a few latent infections may occur. However, variations in the age of sexual maturity among breeds could lead to differences in the age at which brucellosis positivity occurs58.
Sex: Female camels had a greater likelihood of being infected with brucellosis, which may be related to the intrinsic biology of the microorganisms and their tendency to target fetal tissue. Since males with brucellosis may exhibit clinical signs such as epididymitis and orchitis, the prevalence in males could be lower than in females because they may be culled more quickly. In contrast, the higher prevalence in females may be due to the absence of clinical signs such as abortion or metritis in non-pregnant infected females or the lack of observation or identification of abortions in extensive herds64.
Breed: B. abortus is commonly associated with several animal species such as cattle, camels, sheep, swine, dogs, and horses, which may all become infected. In horses, B. abortus can be found in conjunction with Actinomyces bovis, causing conditions such as poll evil and fistulous withers. In addition, B. abortus can infect the mammary gland and regional lymph nodes, leading to bacterial excretion in milk. According to the World Organization for Animal Health, the primary route of transmission is through the placenta, foetal fluids, and vaginal discharges that are released after delivery or abortion, which can result in the release of large numbers of Brucella 65.
Factors such as parity, history of abortion, and contact with other animals can contribute to increased exposure to Brucella infection. There is a correlation between the number of births a female camel has had and the likelihood of testing positive for Brucella infection. Female camels that have given birth more than once are 1.59 times more likely to test positive than those with no history of parturition. Those that have given birth once are 1.25 times more likely to test positive than those with no history of parturition. A higher rate of infection was observed in female camels with multiple births (7.74%) than in those with single parity (0.88%) or no parity (1.01%). Common symptoms of brucellosis in camels include abortion, placental retention, stillbirths, delayed sexual maturity, and infertility 66.
As the number of animals with a history of stillbirths and placental retention was insignificant, the higher seroprevalence observed in camels may be attributed to their rearing with small ruminants (goats) as compared to those with no such contact. A statistically moderate significant association was found between camel groups that had contact with small ruminants and those without such contact. The movement of animals for grazing and watering during the dry season could be a contributing factor to the spread of the disease, as aggregating animals around a watering point could increase the contact between infected and non-infected animals67.
Environmental and climatic factors
During the dry season and parturition time, the probability of brucellosis infection is higher due to increased chances of contamination of the environment by uterus discharge and the survival of the organism in the environment. Environmental factors such as atmospheric conditions and seasons may also influence the spread of the disease, particularly in dry areas with limited water resources where the congregation of mixed ruminants at water points can facilitate the spread of the disease68. The viability of the organism in the environment is enhanced during parturition in the wet season, leading to an increased chance of infecting susceptible animals69. The incidence of brucellosis in the camel population is influenced by breeding and husbandry practices, including herd sizes, animal population density, and poor management, all of which are directly related to the prevalence of the disease32.
Zoonosis importance and Implication in Ethiopia
Human brucellosis remains one of the most common zoonotic diseases in worldwide, which is often referred to as ‘undulant fever’ or ‘Malta fever’ and it also a serious public health problem. A huge number of new cases are annually reported. Infection prevalence in the animal reservoirs determines the incidence of human cases 70. Human brucellosis is mainly an occupational disease, and the main modes of transmission are contacted through skin with animal tissues, blood, urine, vaginal discharge, aborted fetuses and, especially, placentas, and by consuming raw milk and other unheated dairy products Brucella species are also potential agents of bioterrorism and are classified in group B (second-highest priority agent) of the Centers for Disease Control and Prevention (CDC) in the USA. B. melitensis and B. abortus are the two species most found in human cases, and B. melitensis is responsible for the most serious infections. The disease spreads from camels to humans through the milk and or other infected animal products 71. Airborne infections occur in animal pens, stables, laboratories and abattoirs. Some cases have also occurred from accidental self-inoculation with live vaccines. Moreover, it was also shown by Bradenstein et al. 72.
Economic importance
Camels are an essential resource for pastoral communities, providing income, transportation, and various materials such as hides, meat, and milk. They are primarily domestic animals, ensuring food security and serving as draught animals for agriculture and transport. The ability to maintain milk production during dry spells is a significant factor in camel productivity, with households often selling at least one-third of produced milk for cash income73. Milk yield production varies depending on feed availability, with higher production during wet seasons. Camels were the sole means of transportation in arid and semi-arid zones before the arrival of motorized transport. Today, leisure activities such as camel racing and trekking have become popular tourist attractions in some parts of the world74. In Ethiopia, camels are a valuable subset of the country's livestock resources, adapted to harsh environments with limited feed resources10. Their ability to feed on plants that other livestock cannot eat and their economic use of water make them ideal for areas where other livestock may not survive. However, brucellosis is a significant constraint on camel productivity, causing economic losses and impairing public health and socio-economic development. Infected herds experience prolonged inter-calving periods, reduced milk yield, and increased veterinary attendance75. Brucellosis also results in financial losses for people treating the disease, as well as medical costs and lost work hours. This is particularly impactful for livestock owners, who represent a vulnerable segment of rural communities. The economic losses associated with brucellosis are significant and affect various aspects of the livestock industry, including export trade and government research and eradication programs10.
Prevention and Control
Controlling and eradicating diseases in pastoral areas can be difficult due to management practices, such as keeping infected animals with healthy ones. In regions where brucellosis is prevalent in livestock, many cases of camel brucellosis are found. While brucellosis has been eradicated in some countries, it remains widespread and economically significant in developing countries. Strategic options, including control and eradication measures, can be implemented to reduce the prevalence of the disease to an acceptable level and eliminate infection foci76. However, efficient animal disease surveillance is a necessary prerequisite for any control program. Whole-herd vaccination is most effective in low-prevalence countries, while test-and-slaughter followed by vaccination is recommended in high-prevalence countries5. It is essential to castrate all Brucella-positive bulls, avoid breeding positive females, and vaccinate to control the spread of the disease. To prevent infection, vaccines have been developed, and whole-herd vaccination is the most effective means of elevating animal immunity. Live and killed vaccines, such as B. abortus S-19, B. melitensis, B. melitensis strain M111, B. abortus strain RB51, B. abortus 45/20, and B. melitensis H.38, are available and have their own advantages and disadvantages25.
Immune Response
Once the Brucella organism enter to body of animal and cause infection. Humoral and cell-mediated immune response induce during Brucella infection. The magnitude and duration of these responses can be affected by many factors including virulence of the infecting strain, size of inoculum, age, sex, pregnancy, species, and immune status of the host. Although humoral immune response plays an important role in immunity to Brucella, it is the cell-mediated response that is most important in providing protection77.
Humoral immune response
IgM is the first immunoglobulin produced after initial strain 19 immunization, followed by IgG. IgG1 is the most abundant and its concentration exceeds that of IgG2. Age at immunization and number of organisms administered affect the antibody response magnitude and duration. After standard dose immunization, IgG antibody concentrations decline to diagnostically insignificant levels over 3-6 months, with residual antibody predominantly IgM. A minimum dose may take 2-7 months to develop "reactor" titres, while a large dose produces significant agglutinin titre in 2-4 weeks. Most infected animals develop a diagnostic agglutinin titre 30-60 days after exposure, but there is a great variation in response from animal to animal. Infected animals may not produce IgG antibodies until parturition, making it difficult to differentiate from non-infected vaccinated animals. IgM is the most efficient antibody in the tube agglutination test, while IgG1 has the capacity to block agglutination by other isotypes77.
Cellular immune response
Brucella bacteria produce antibodies to survive in macrophage cells. Virulent strains of Brucella can survive in normal macrophages for long periods, making recovery from infection dependent on increased bactericidal activity of phagocytic cells. T-lymphocytes release lymphokines to activate macrophages. Brucella antigens recognized by T-cells stimulate antibody release. Cell-mediated immunity is induced by live organisms capable of establishing persistent intracellular infection and certain types of antigens. The role of cytotoxic cells in the immune response to Brucella is unknown. More studies are needed to understand protective immunity to Brucella 78.
Conclusion
Camels in Ethiopia are essential for pastoralists, but their productivity is affected by various diseases, including Brucellosis. The disease's prevalence is on the rise globally and requires urgent intervention to prevent further spread. Brucellosis has severe consequences for both animal and human health, and eradication can only be achieved through control, prevention, and surveillance. Risk factors for the disease include age, parity, herd size, and inadequate vaccination. Awareness campaigns, vaccination programs, and establishment of suitable laboratories are recommended. To minimize occupational and public health risks, people should avoid consuming unpasteurized dairy products, and proper disposal of aborted fetuses and contaminated materials is necessary.
Acknowledgments
The authors extend gratitude to Hawassa University Faculty of Veterinary Medicine for providing materials and internet used to write this manuscript.
Authorship contribution statement
WW: Writing and review the manuscript. SAN: Conceptualizing and editing edit the manuscript. All authors have read and approved the manuscript.
Funding
Not applicable
Availability of data and materials
The data supporting the findings are presented in the manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors are consented for the publication of the manuscript.
Abbreviations
AMOS-B. abortus, melitensis, ovis, suis
CDC-Center for Disease Control
CFT-Complement Fixation test
CSA-Central Statically Authority
ELISA-Enzyme linked immuno sorbent assay
FAO-Food and Agriculture Organization
IgG-Imminoglobin G
IgM-Imminoglobin M
LPS-Lipopolysaccharide
OIE-Office international Epizootics
PCR-Polymerase Chine Reaction
RBT-Rose bangle test
R-LPS-Rough Lipopolysaccharide
S-LPS-Smooth Lipopolysaccharide
USA-United State America
WHO-World health organization
References
- 1.Alamian S, Dadar M.Brucella abortus contamination of camel milk in two Iranian regions. Preventive veterinary medicine. 2019, 104708.
- 2.Behnke R. (2010) The contribution of livestock to the economies of IGAD member states: study findings, application of the methodology in Ethiopia and recommendations for further work. IGAD LPI Working Paper 02–10.
- 3.Biffa D. (2002) editor Camel and the changing system of Borena pastoral production. Proceeding of the Annual Conference of the Ethiopian Veterinary Association (EVA), Addis Ababa , Ethiopia .
- 4.Farah Z, Fischer A. (2004) An introduction to the camel. Milk and Meat from the Camel Handbook on Products and Processing 15-22.
- 6.Köhler-Rollefson I, Mundy P, Mathias E. (2001) A field manual of camel diseases: traditional and modern health care for the dromedary: ITDG publishing;.
- 8.Kuplulu O, Sarimehmetoglu B.Isolation and identification of Brucella spp. in ice cream. , Food Control 15(7), 511-4.
- 9.Musa M, Eisa M, El Sanousi EM, Wahab M A, Perrett L.Brucellosis in camels (Camelus dromedarius) in Darfur, western Sudan. , Journal of Comparative Pathology 138(2), 151-5.
- 10.Jafer M, Mengistu D, Eshetu A, Belina D. (2018) . , SERO-PREVALENCE OF BRUCELLOSIS IN CAMELS AND FEBRILE HUMAN PATIENTS ATTENDING HEALTH FACILITIES IN SELECTED DISTRICTS OF EASTERN ETHIOPIA
- 11.Abebe G, Worku Y, Mamo G, Nazir S.Sero-prevalence and associated risk factors of brucellosis in camel at Akaki Abattoir, Central Ethiopia. , Journal of Animal Research 7(4), 617-22.
- 12.Schelling E, Diguimbaye C, Daoud S, Nicolet J, Boerlin P et al.Brucellosis and Q-fever seroprevalences of nomadic pastoralists and their livestock in Chad. Prev Vet Med. Epub 2003/11/19. doi: 10.1016/j.prevetmed.2003.08.004. PubMed PMID: 61(4), 279-93.
- 13.Skalsky K, Yahav D, Bishara J, Pitlik S, Leibovici L et al.Treatment of human brucellosis: systematic review and meta-analysis of randomised controlled trials. , Bmj 336(7646), 701-4.
- 14.Sprague L D, Al-Dahouk S, Neubauer H.A review on camel brucellosis: a zoonosis sustained by ignorance and indifference. Pathogens and global health. 106(3), 144-9.
- 15.Hadush A, Pal M.Brucellosis-An infectious re-emerging bacterial zoonosis of global importance. , Int J Livest Res 3(1), 28-34.
- 16.Hadush A, Pal M, Kassa T, Zeru F.Sero-epidemiology of camel brucellosis in the Afar region of Northeast Ethiopia. , Journal of Veterinary Medicine and Animal Health 5(9), 269-75.
- 17.Tilahun B, Bekana M, Belihu K, Zewdu E.Camel brucellosis and management practices in Jijiga and Babile districts. , Eastern Ethiopia, Journal of Veterinary Medicine and Animal Health 5(3), 81-6.
- 18.Neja S A, Gari Y.Study on the Major Cattle Health and Production Constraints in and Around Haramaya Town. , Ethiopia. Journal of Biology, Agriculture and Healthcare 2020, 1-9.
- 19.Adam A, Neja S. (2022) Assessment of Veterinary Extension Service And Public Perception on Zoonotic Disease at Robe Veterinary Clinic of Bale Zone. , Oromia, Ethiopia, J Vet Heal Sci 3(4), 373-383.
- 21.El-Sayed A, Awad W.Brucellosis: Evolution and expected comeback. International journal of veterinary science and medicine. 2018-6.
- 22.Whatmore A M.Current understanding of the genetic diversity of Brucella, an expanding genus of zoonotic pathogens. , Infection, Genetics and Evolution 9(6), 1168-84.
- 23.CfD Control. (2022) Prevention. CDC and Texas health officials warn about illness linked to raw milk from Texas Dairy.(Cited 21.
- 24.Robinson A, Production A. (2003) Guidelines for coordinated human and animal brucellosis surveillance: FAO. , Rome, Italy:;
- 25.Thakur S, Thapliyal D.Seroprevalence of brucellosis in man. , Journal of communicable diseases 34(2), 106-9.
- 26.Emmerzaal A, J De Wit, Dijkstra T, Bakker D, F Van Zijderveld.The Dutch Brucella abortus monitoring programme for cattle: The impact of false‐positive serological reactions and comparison of serological tests. Veterinary quarterly. 24(1), 40-6.
- 27.Seleem M N, Boyle S M, Sriranganathan N.Brucellosis: a re-emerging zoonosis. Veterinary microbiology. 2010-140.
- 28.G-B B. (2014) Brucellosis An emerging disease with public health implications?. Symposium “Exotic livestock diseases – a Swedish issue?” SLU / CGD–Uppsala – Sweden - 12 .
- 30.Damir H A, Tageldin M, Kenyon S, Idris O.Isolation of Brucella abortus from experimentally infected dromedary camels in Sudan: a preliminary report. Veterinary Research Communications. 1989, 403-6.
- 31.Nada A, Ahmed W.Investigation on brucellosis in some genital abnormalities of she-camels (Camelus deomedarius). , INTERNATIONAL JOURNAL OF ANIMAL SCIENCES 1993, 37.
- 32.Wernery U, Thomas R, Syriac G, Raghavan R, Kletzka S.Seroepidemiological studies for the detection of antibodies against nine infectious diseases in dairy dromedaries (Part-I). , Journal of Camel Practice and Research 14(2), 85-90.
- 33.Quinn P, Markey B, Carter M, Donnely W, Lonard F et al. (2002) . Brucella Species in Veterinary Microbiology and Microbial Disease. Londen, Black Well Science ltd 999-1000.
- 34.Morata P, Queipo-Ortuno M I, Reguera J M, García-Ordonez M A, Cárdenas A et al.Development and evaluation of a PCR-enzyme-linked immunosorbent assay for diagnosis of human brucellosis. , Journal of clinical microbiology 41(1), 144-8.
- 35.Radwan A, Bekairi S, Mukayel A, Al-Bokmy A, Prasad P et al.Control of Brucella melitensis infection in a large camel herd in Saudi Arabia using antibiotherapy and vaccination with Rev. 1 vaccine. Revue scientifique et technique (International Office of Epizootics). 14(3), 719-32.
- 36.Omer M, Musa M, Bakhiet M, Perrett L.Brucellosis in camels, cattle and humans: associations and evaluation of serological tests used for diagnosis of the disease in certain nomadic localities in Sudan. Revue scientifique et technique (International Office of Epizootics). 29(3), 663-9.
- 37.Ron-Román J, Ron-Garrido L, Abatih E, Celi-Erazo M, Vizcaíno-Ordóñez L et al.Bayesian Evaluation of Three Serological Tests for Detecting Antibodies against Brucella spp. among Humans in the Northwestern Part of Ecuador. PubMed PMID: 31038099; PubMed Central PMCID: PMCPMC6553909 , Am J Trop Med Hyg 100(6), 1312-20.
- 39.Mantur B, Amarnath S, Shinde R.Review of clinical and laboratory features of human brucellosis. Indian journal of medical microbiology. 25(3), 188-202.
- 40.Rojas X, Muñoz S, Otto B, Pérez B, Nielsen K.The use of polarized fluorescence assay (PF) and competitive ELISA test (c-ELISA) for the diagnosis of brucellosis in South American camelids. , ARCHIVOS DE MEDICINA VETERINARIA 36(1), 59-64.
- 41.Nielsen H, Ewalt R. (2004) Bovine brucellosis: In manual of standards for diagnostic tests and vaccines,(5th Edn) OIE. , Paris, France 328-45.
- 42.Mammeri A, Kayoueche F, Benmakhlouf A.Peri-urban breeding practice of one-humped camel (Camelus dromedarius) in the Governorate of Biskra (Algeria); A new option. , J Anim Prod Adv 4(5), 403-15.
- 43.CSA.Report on monthly average retail prices of goods and services in rural areas by Killil and Zone;. , Statistical Bulletin, CSA, Addis Ababa, Ethiopia 222(1), 268.
- 44.Solomon A. (2003) Authority ELM. Livestock marketing in Ethiopia: a review of structure, performance, and development initiatives.
- 45.Tegegne A. (2009) Transhumance cattle production system in North Gondar. Working Paper (International Livestock Research Institute Improving Productivity and Market Success of Ethiopian Farmers Project); no 14 , Amhara Region, Ethiopia .
- 46.Awole M, Gebre-Selassie S, Kassa T, Kibru G.Isolation of potential bacterial pathogens from the stool of HIV-infected and HIV-non-infected patients and their antmicrobial susceptibility patterns in Jimma Hospital, south west Ethiopia. , Ethiopian Medical Journal 40(4), 353-64.
- 47.MMAS Gwida. (2010) Isolation, identification and typing of Brucella species as zoonotic pathogens by using conventional and molecular biological methods.
- 48.Teshome H, Molla B, Tibbo M.A seroprevalence study of camel brucellosis in three camel-rearing regions of Ethiopia. Tropical Animal Health and Production. 2003, 381-90.
- 49.Megersa B, Biffa D, Abunna F, Regassa A, Godfroid J et al.Seroepidemiological study of livestock brucellosis in a pastoral region. , Epidemiology & Infection 140(5), 887-96.
- 50.Birhanu T. (2006) Camel management and status of camel brucellosis in Jijiga zone, southeast lowland areas in Somali national regional state, eastern Ethiopia: MSc Thesis, Faculty of Veterinary Medicine, Addis Ababa University, Debre …;.
- 51.Mohammed O, Megersa B, Abebe R, Abera M, Regassa A et al.Seroprevalence of brucellosis in camels in and around Dire Dawa city, Eastern Ethiopia. Journal of animal and veterinary advances. 10(9), 1177-83.
- 52.Zewolda S W, Wereta M H.Seroprevalence of Brucella infection in camel and its public health significance in selected districts of Afar region. , Ethiopia. Journal of Environmental and Occupational Health 1(2), 91-8.
- 53.Gumi B, Firdessa R, Yamuah L, Sori T, Tolosa T et al.Seroprevalence of brucellosis and Q-fever in southeast Ethiopian pastoral livestock. , Journal of veterinary science & medical
- 54.Gessese A, Mulate B, Nazir S, Asmare A.Seroprevalence of brucellosis in camels (Camelus dromedaries) in South East Ethiopia. , Journal of Veterinary Scientific Medical Diagnosis 3(1), 2.
- 55.Bekele W A, Tessema T S, Melaku S K.Camelus dromedarius brucellosis and its public health associated risks in the Afar National Regional State in northeastern Ethiopia. Acta veterinaria scandinavica. 55(1), 1-8.
- 56.Zeru F, Gebrezgabher W, Dessalegn K, Tilahun S, Guben Y et al.Prevalence and risk factor of brucellosis in dromedaries in selected pastoral districts of afar. , Northeastern Ethiopia. Prevalence 6(1).
- 57.Warsame I, Alemu S, Temesgen W, Molla W. (2020) Seroprevalence and associated risk factors of camel (Camelus dromedaries) brucellosis in and around Dire Dawa. , Ethiopia
- 58.Megersa B, Biffa D, Abunna F, Regassa A, Godfroid J et al.Seroprevalence of brucellosis and its contribution to abortion in cattle, camel, and goat kept under pastoral management in Borana, Ethiopia. Tropical animal health and production. 2011, 651-6.
- 59.Admasu P. (2017) Seroprevalence of camel brucellosis in Yabello district of borena zone, southern Ethiopia. , Journal of Veterinary Medicine and Research
- 60.Paridah M, Moradbak A, Mohamed F, Owolabi T, Abdulwahab M et al.Risk factors for Brucella spp. Domestic and Wild Animals. 2016, 1-12.
- 61.Radostitis O, Gay C, Blood D Hinchcliff.Veterinary medicine, A text of the diseases of cattle, horses, sheep, pigs, and goats, 10th ed. 966-98.
- 62.Al-Majali A M, Al-Qudah K M, Al-Tarazi Y H, Al-Rawashdeh O F.Risk factors associated with camel brucellosis in Jordan. Tropical Animal Health and Production. 2008, 193-200.
- 63.Banai M.Control of small ruminant brucellosis by use of Brucella melitensis Rev. 1 vaccine: laboratory aspects and field observations. Veterinary microbiology. 2002-90.
- 64.Salisu U, Kudi C, Bale J, Babashani M, Kaltungo B et al.. Seroprevalence of Brucella antibodies in camels in Katsina State, Nigeria. Tropical Animal Health and Production 2017, 1041-6.
- 66.Robayo Y, Esubalew S.Seroprevalence and Associated Risk Factors of Brucellosis in Camels Kept Under Pastoral Management in Fafen Zone, Somali Regional State. , Ethiopia. Int J Livest Res 7(1).
- 67.Waktole H, Aden M, Ashenafi H.Seroepidemiology of camel brucellosis in and around Dire Dawa, Eastern Ethiopia. Veterinary Medicine International. 2022-2022.
- 68.McDermott J J, Arimi S.Brucellosis in sub-Saharan Africa: epidemiology, control and impact. Veterinary microbiology. 2002-90.
- 69.Baumann M, Zessin K.Productivity and health of camels (Camelus dromedarius) in Somalia: associations with trypanosomosis and brucellosis. Tropical Animal Health and Production. 1992, 145-56.
- 70.D Von Hieber. (2010) Investigation of occurrence and persistence of brucellosis in female camel dams (Camelus dromedarius) and their calves: Thesis, Universität Ulm. , Germany;
- 71.Greenfield R A, Drevets D A, Machado L J, Voskuhl G W, Cornea P et al.Bacterial pathogens as biological weapons and agents of bioterrorism. The American journal of the medical sciences. 323(6), 299-315.
- 72.Bardenstein S, Mandelboim M, Ficht T A, Baum M, Banai M.Identification of the Brucella melitensis vaccine strain Rev. 1 in animals and humans in Israel by PCR analysis of the Pst I site polymorphism of its omp2 gene. , Journal of clinical microbiology 40(4), 1475-80.
- 73.Getahun T, Bruckner H, editors. (2000) Camel milk and meat utilization. in eastern Ethiopia. Conference of Ethiopian Society of Animal Production, 8, Addis Abeba (Ethiopia) 24-26.
- 75.Al-Majali A M.Seroepidemiology of caprine brucellosis in Jordan. , Small Ruminant Research 58(1), 13-8.
- 76.Blasco J M, Molina-Flores B.Control and eradication of Brucella melitensis infection in sheep and goats. Veterinary Clinics: Food Animal Practice. 27(1), 95-104.